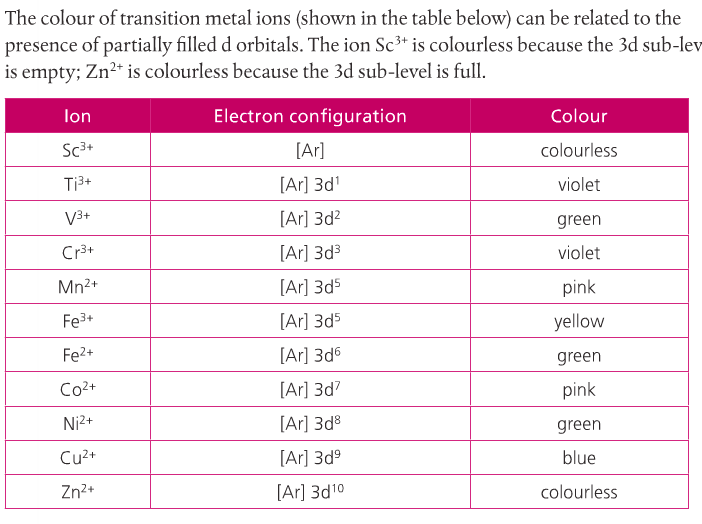
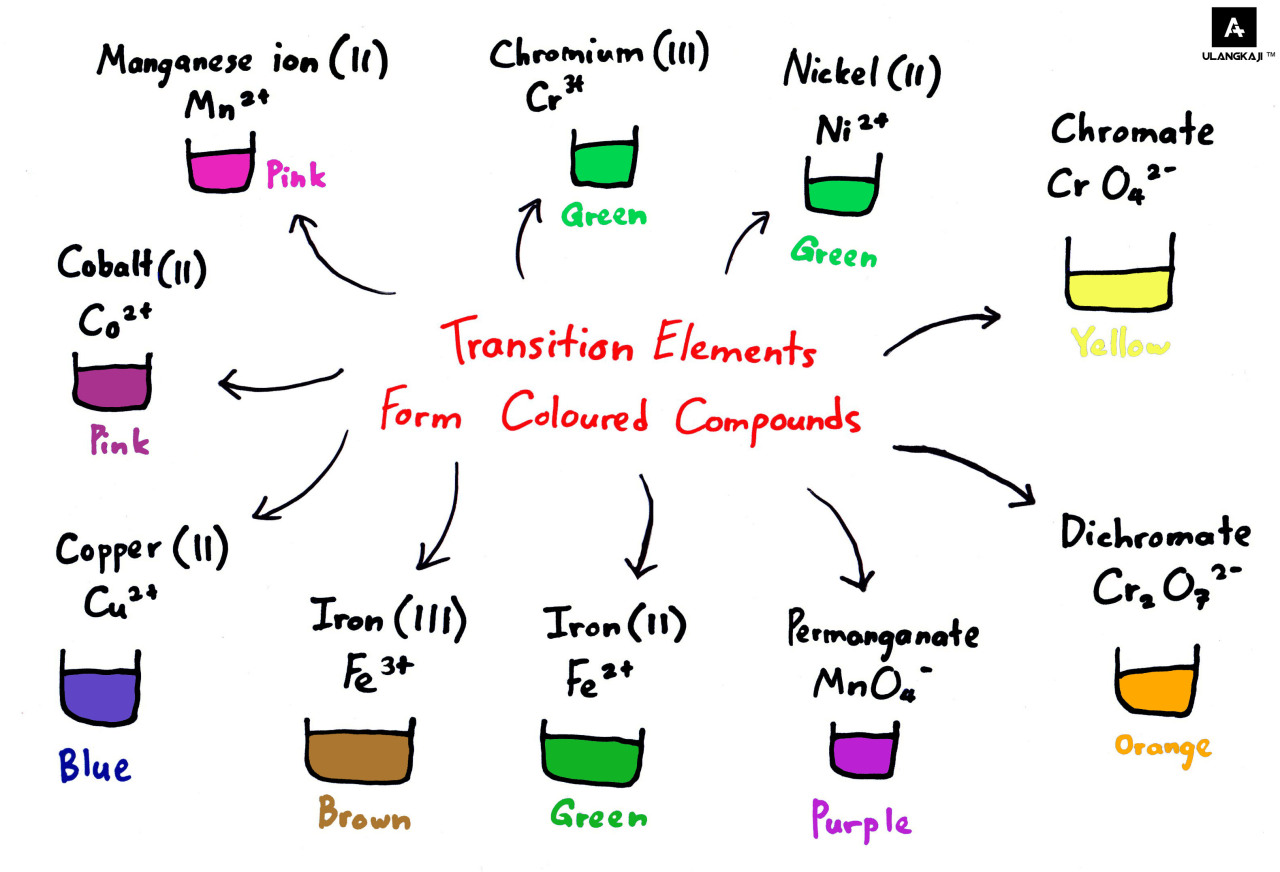
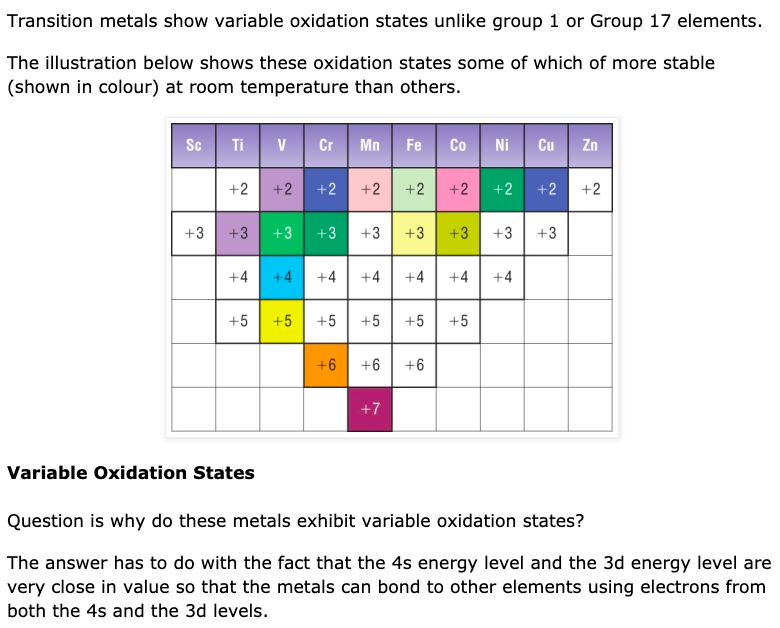
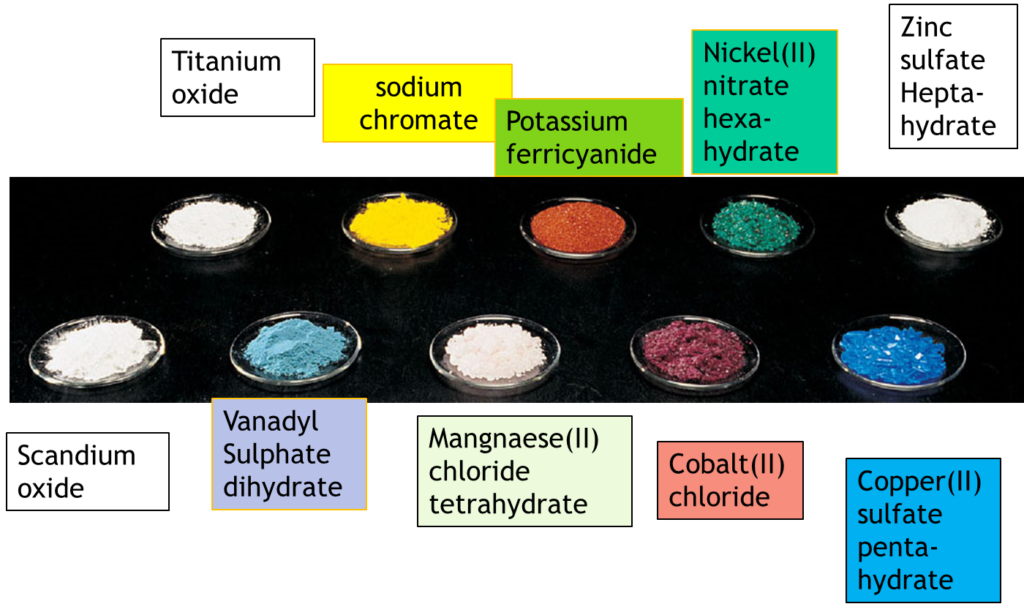
Why Transition Metals Form Coloured Compounds - In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
13 Transition Metals & Colored Complexes The!Mad!Scientist!
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
SPMStraightA — Transition Metals make coloured compounds
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
PPT Atoms and ElementsThe Nature of Matter PowerPoint Presentation
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Explain how transition elements form coloured compounds?
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
13 Transition Metals & Colored Complexes The!Mad!Scientist!
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Why do Transition Metals Form Coloured Compounds? YouTube
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
The transition metals generally form coloured compounds. Transition meta..
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Transition metals W3schools
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Transition Metals as Colored Compounds
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
In Their Lower Oxidation States, The Transition Elements Form Ionic Compounds;
In their higher oxidation states, they form covalent.