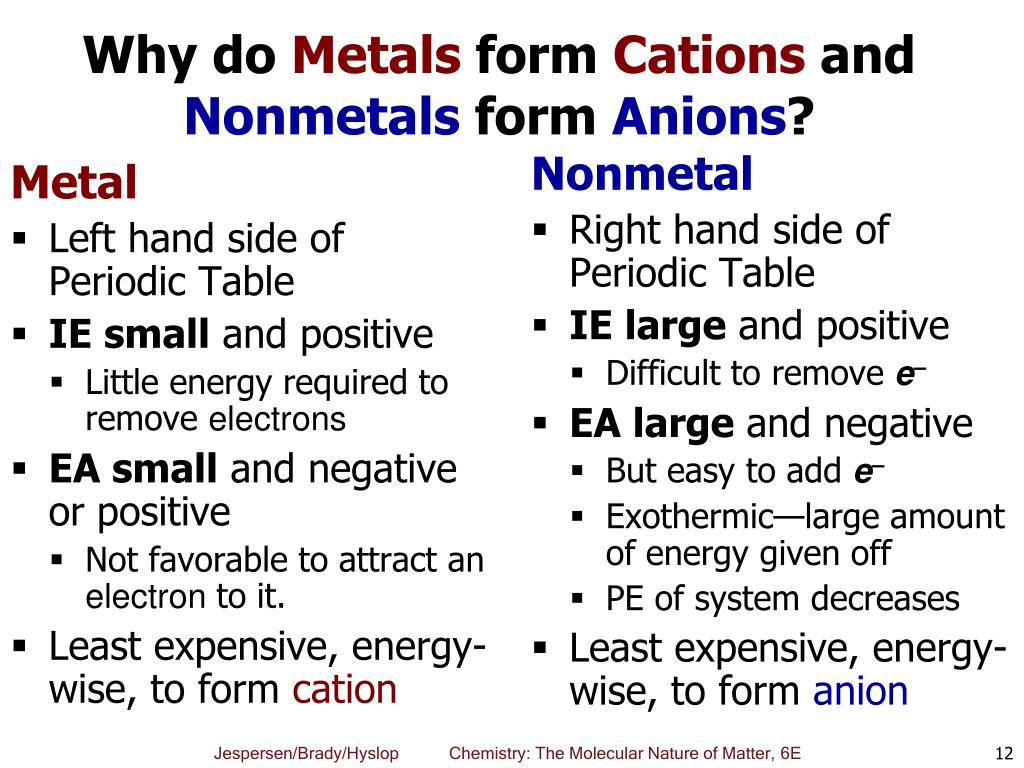
Why Do Metals Form Cations - Metals are prone to losing electrons as a result of the arrangement of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. In more detail, the formation of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Cations are formed when a neutral atom loses an electron.
Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a result of the arrangement of. In more detail, the formation of.
Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a result of the arrangement of. In more detail, the formation of. Cations are formed when a neutral atom loses an electron.
Do Metals Form Anions Or Cations
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. Cations are formed when a neutral atom loses an electron. Metals are.
Which Metals Form Cations With Varying Positive Charges TerryhasYoder
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. In more detail, the formation of. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive.
Metals Tend to Form Cations or Anions IndiahasPeck
Metals are prone to losing electrons as a result of the arrangement of. Cations are formed when a neutral atom loses an electron. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. First, each element that forms a cation is a metal, except for one.
Solved Question 6 (1 point) Why do metals tend to form
Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Cations are formed when a.
What are the properties of most metals? ppt download
Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one.
Explain why almost all metals tend to form cations. Numerade
In more detail, the formation of. Metals are prone to losing electrons as a result of the arrangement of. Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals form positive ions because they tend.
Ionic Compounds Stone Cold Chemistry Talk
Metals are prone to losing electrons as a result of the arrangement of. Cations are formed when a neutral atom loses an electron. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. First, each element that forms a cation is a metal, except for one.
Cations and Anions Definitions, Examples, and Differences
Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Metals are prone to losing electrons as a result of the arrangement of. Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Cations are formed when a neutral atom loses an electron. In more detail, the formation of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are.
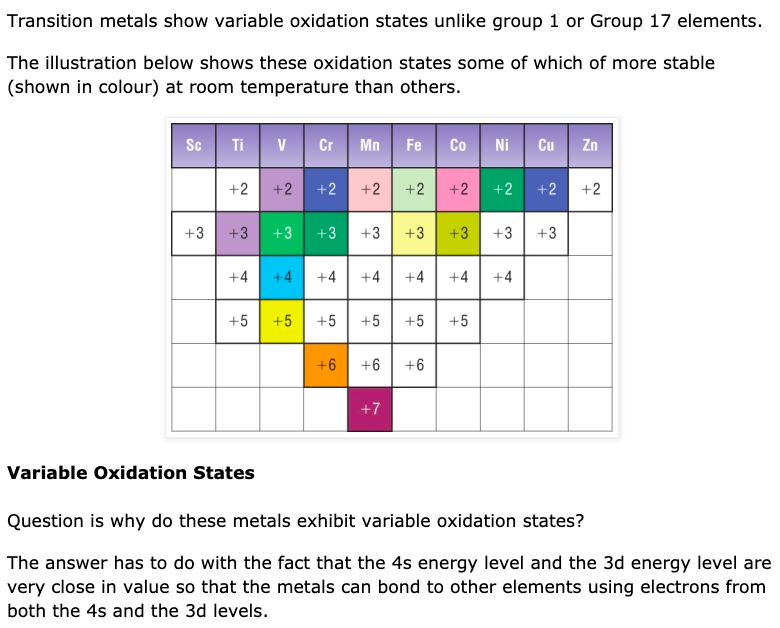
13 Transition Metals & Colored Complexes The!Mad!Scientist!
Metals are prone to losing electrons as a result of the arrangement of. In more detail, the formation of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a.
Metals Are Prone To Losing Electrons As A Result Of The Arrangement Of.
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Cations are formed when a neutral atom loses an electron. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of.

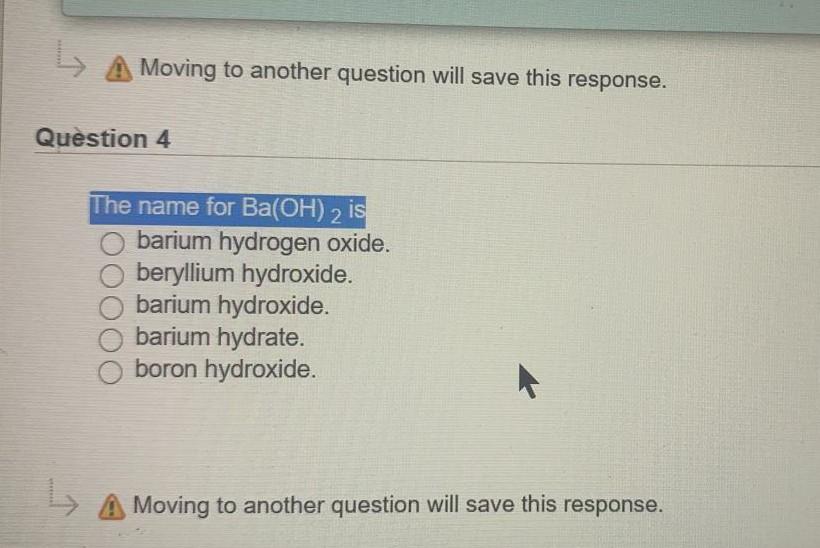
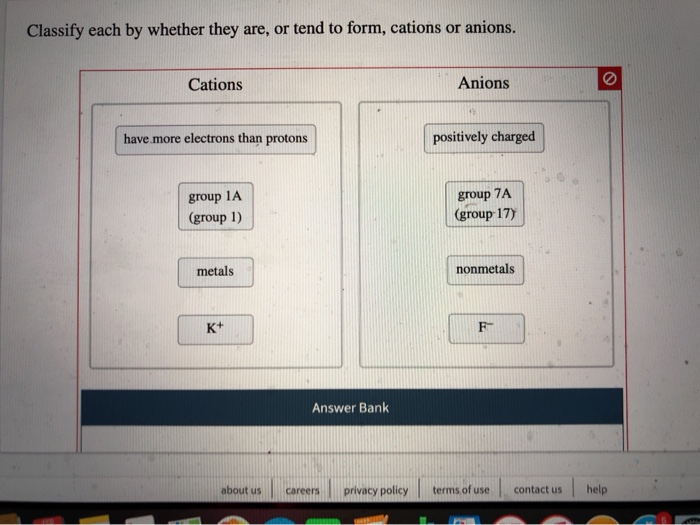

.jpg)