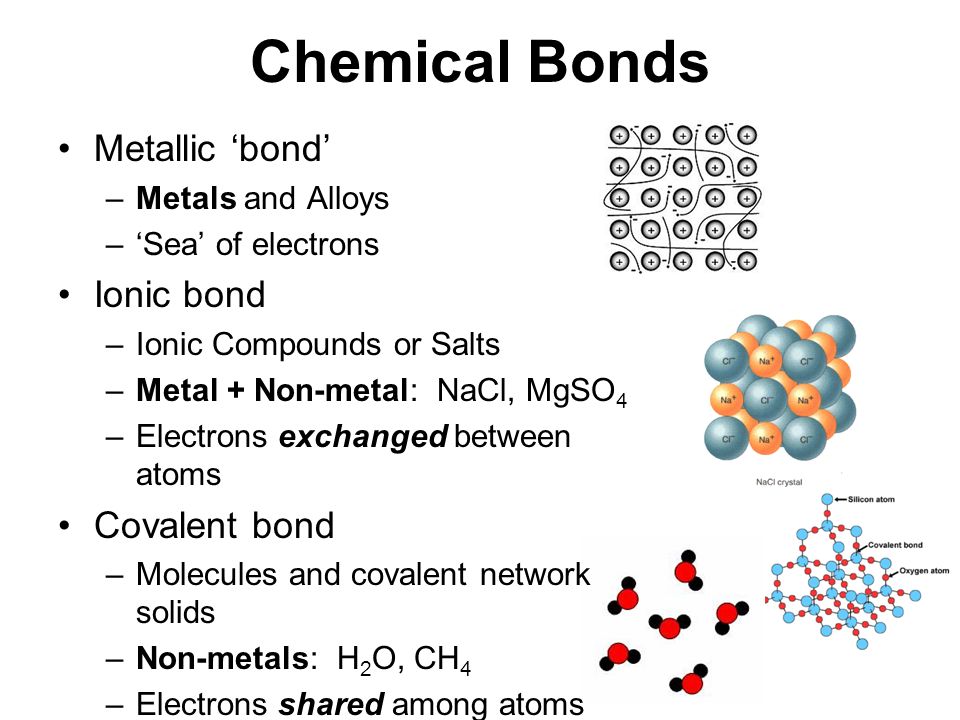
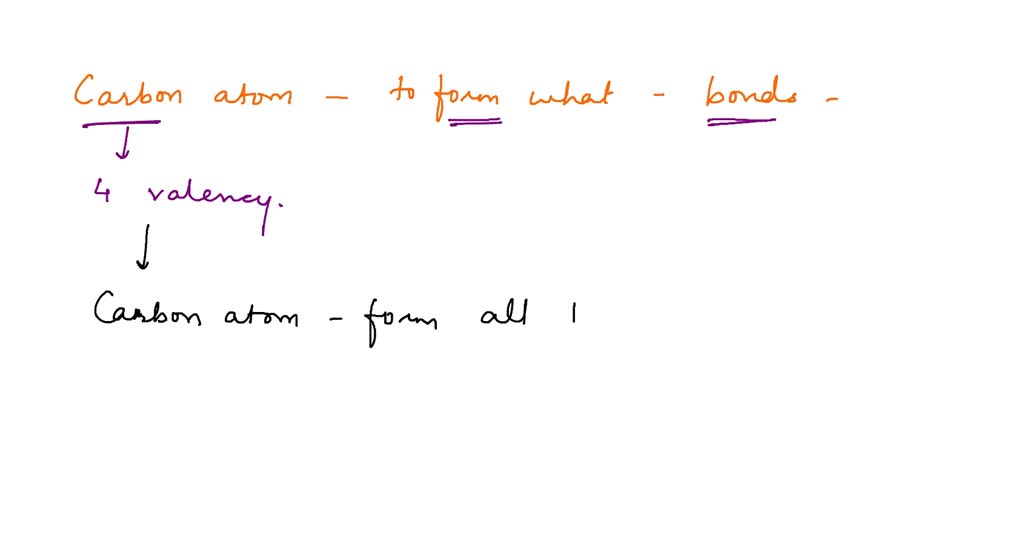Which Atom Is Most Likely To Form A Metallic Bond - Aluminum is a metal known for its ability to readily donate its valence. Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Among the given options, aluminum (al) is the most likely to form a metallic bond. Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Aluminum (al) is a metal and is known for its ability to form metallic. Electrons are shared between the positive ions called cations. Which atom is most likely to form a metallic bond? They involve the sharing of free electrons among a lattice of metal atoms. Metallic bonds are the kind of bonds that forms between metallic atoms.
Among the given options, aluminum (al) is the most likely to form a metallic bond. Metallic bonds are the kind of bonds that forms between metallic atoms. Electrons are shared between the positive ions called cations. Aluminum (al) is a metal and is known for its ability to form metallic. They involve the sharing of free electrons among a lattice of metal atoms. Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Aluminum is a metal known for its ability to readily donate its valence. Which atom is most likely to form a metallic bond? Metallic bonds are forces of attraction between positive metal ions and the valence electrons that.
Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Aluminum (al) is a metal and is known for its ability to form metallic. They involve the sharing of free electrons among a lattice of metal atoms. Among the given options, aluminum (al) is the most likely to form a metallic bond. Electrons are shared between the positive ions called cations. Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Aluminum is a metal known for its ability to readily donate its valence. Which atom is most likely to form a metallic bond? Metallic bonds are the kind of bonds that forms between metallic atoms.
A brief history of the atom The Techno Circuit
Electrons are shared between the positive ions called cations. Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Among the given options, aluminum (al) is the most likely to form a metallic bond. Aluminum is a metal known for its ability to readily donate its valence. They involve.
In a metallic bond, metal atom loses its valence electrons to form.........
Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Electrons are shared between the positive ions called cations. Among the given options, aluminum (al) is the most likely to form a metallic bond. Aluminum (al) is a metal and is known for its ability to form metallic. Metallic bonds involve many valence electrons shared.
Untitled Document [www.aml.engineering.columbia.edu]
Metallic bonds are the kind of bonds that forms between metallic atoms. Aluminum is a metal known for its ability to readily donate its valence. Among the given options, aluminum (al) is the most likely to form a metallic bond. Aluminum (al) is a metal and is known for its ability to form metallic. Metallic bonds involve many valence electrons.
SOLVED A carbon atom is most likely to form what kind of bond(s) with
Among the given options, aluminum (al) is the most likely to form a metallic bond. Metallic bonds are the kind of bonds that forms between metallic atoms. Aluminum is a metal known for its ability to readily donate its valence. Aluminum (al) is a metal and is known for its ability to form metallic. Metallic bonds are forces of attraction.
Exploring Metallic Bonding Definition, Properties, Examples
They involve the sharing of free electrons among a lattice of metal atoms. Among the given options, aluminum (al) is the most likely to form a metallic bond. Electrons are shared between the positive ions called cations. Aluminum is a metal known for its ability to readily donate its valence. Metallic bonds are the kind of bonds that forms between.
Which one of the atoms shown would most likely form a cation with a
Aluminum is a metal known for its ability to readily donate its valence. Which atom is most likely to form a metallic bond? Among the given options, aluminum (al) is the most likely to form a metallic bond. Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Metallic bonds are the kind of bonds.
Solved In each of the molecules drawn below one chemical
Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Aluminum is a metal known for its ability to readily donate its valence. Electrons are shared between the positive ions called cations. Metallic bonds are the kind of bonds that forms between metallic atoms. Aluminum (al) is a metal.
Chemistry B.Sc Level How many types of chemical bond
Aluminum (al) is a metal and is known for its ability to form metallic. Aluminum is a metal known for its ability to readily donate its valence. Metallic bonds are the kind of bonds that forms between metallic atoms. Electrons are shared between the positive ions called cations. Metallic bonds involve many valence electrons shared by many atoms, so the.
Metallic Bonding Chemistry Definition DEFINITION GHW
Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Which atom is most likely to form a metallic bond? Aluminum is a metal known for its ability to readily donate its valence. Among the given options, aluminum (al) is the most likely to form a metallic bond. Metallic bonds involve many valence electrons shared.
Metallic Bond — Formation & Compounds Expii
Electrons are shared between the positive ions called cations. Which atom is most likely to form a metallic bond? Metallic bonds are the kind of bonds that forms between metallic atoms. Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Aluminum (al) is a metal and is known.
Aluminum Is A Metal Known For Its Ability To Readily Donate Its Valence.
Metallic bonds involve many valence electrons shared by many atoms, so the bonds can move around as the metal is pounded. Which atom is most likely to form a metallic bond? Metallic bonds are forces of attraction between positive metal ions and the valence electrons that. Metallic bonds are the kind of bonds that forms between metallic atoms.
They Involve The Sharing Of Free Electrons Among A Lattice Of Metal Atoms.
Electrons are shared between the positive ions called cations. Aluminum (al) is a metal and is known for its ability to form metallic. Among the given options, aluminum (al) is the most likely to form a metallic bond.
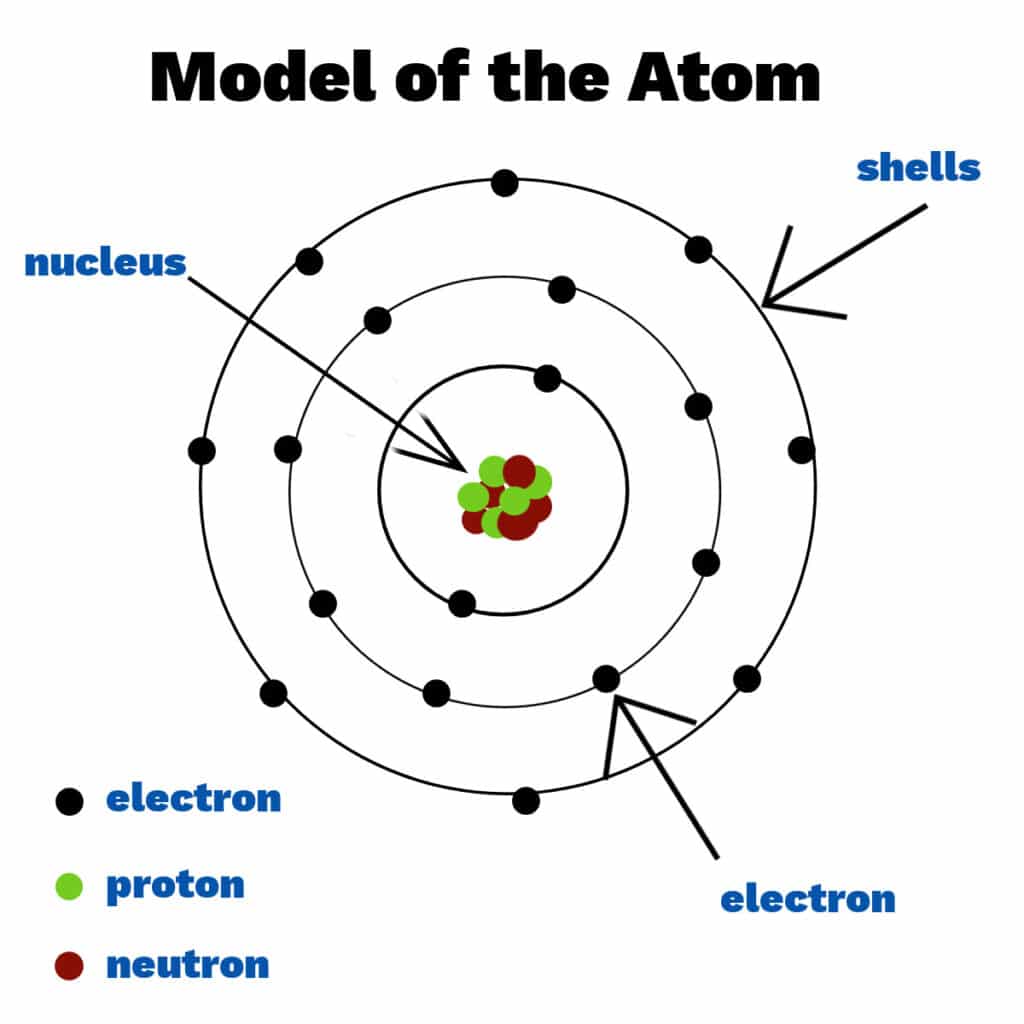

![Untitled Document [www.aml.engineering.columbia.edu]](http://www.aml.engineering.columbia.edu/ntm/images/atomic.jpg)