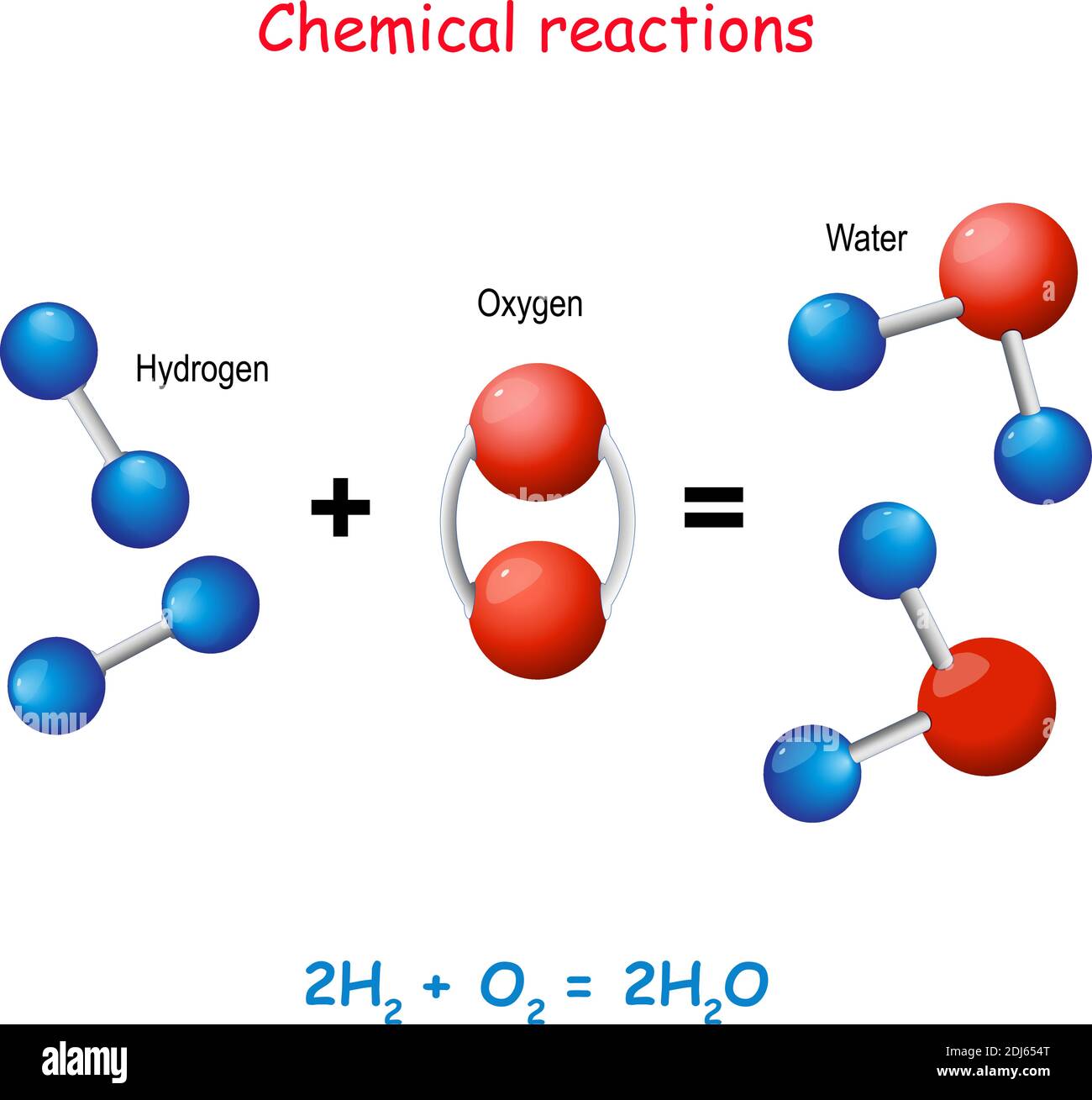
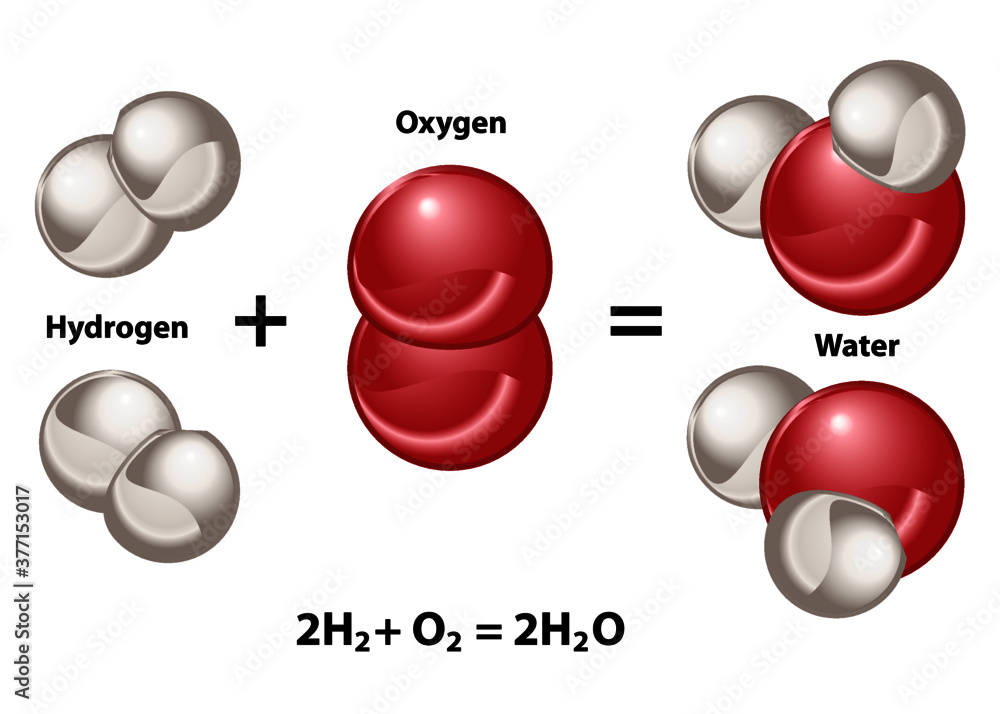
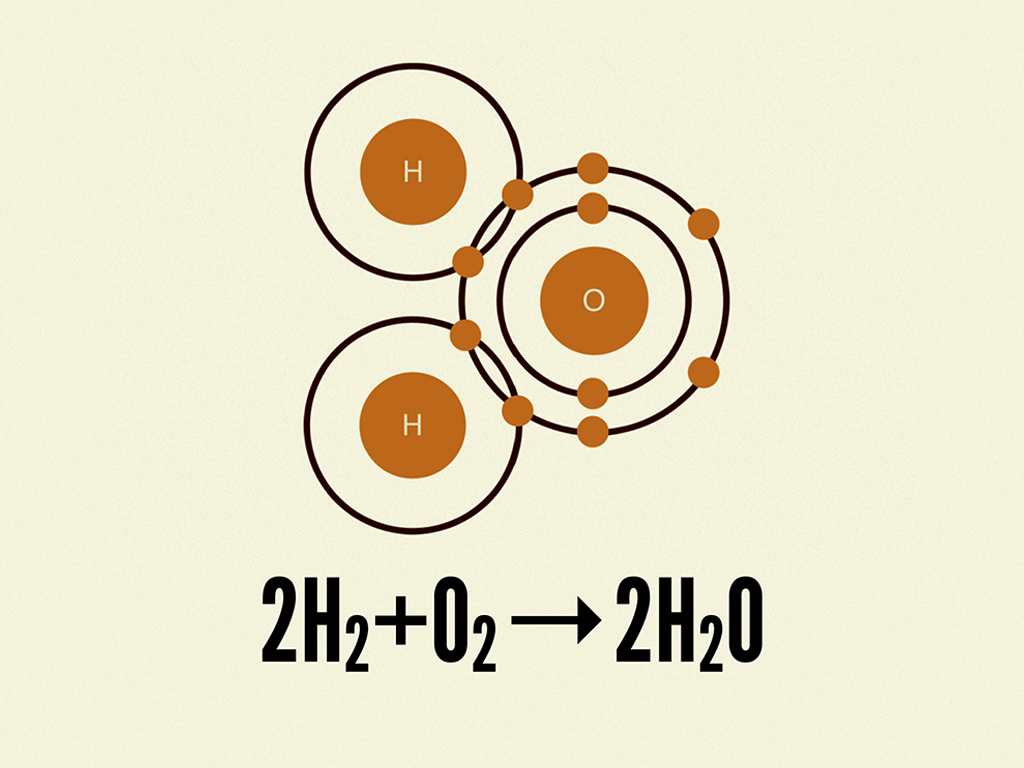
When Hydrogen And Oxygen Combine And Form Water Water Is - Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen.
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water.
Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and.
learning through art water molecules and hydrogen bonding share4uhoshiro
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule.
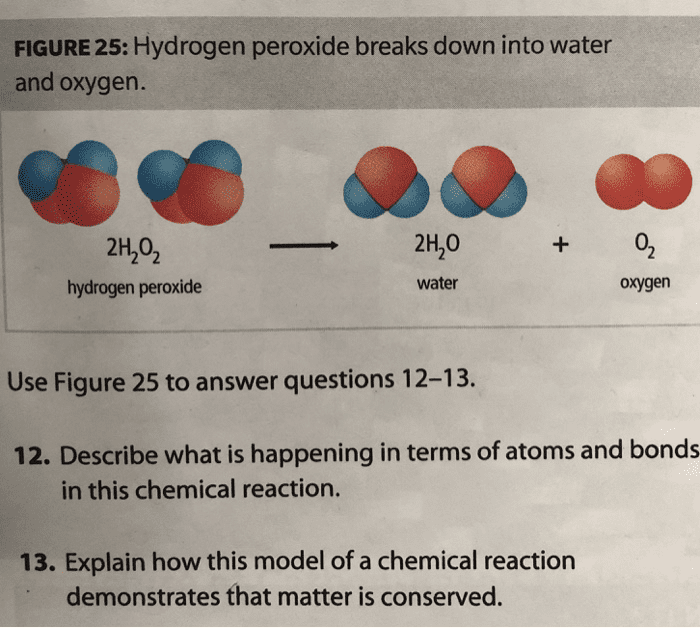
OneClass FIGURE 25 Hydrogen peroxide breaks down into water and
Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to.
Water Molecule. Oxygen And Hydrogen Cartoon Vector
While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them.
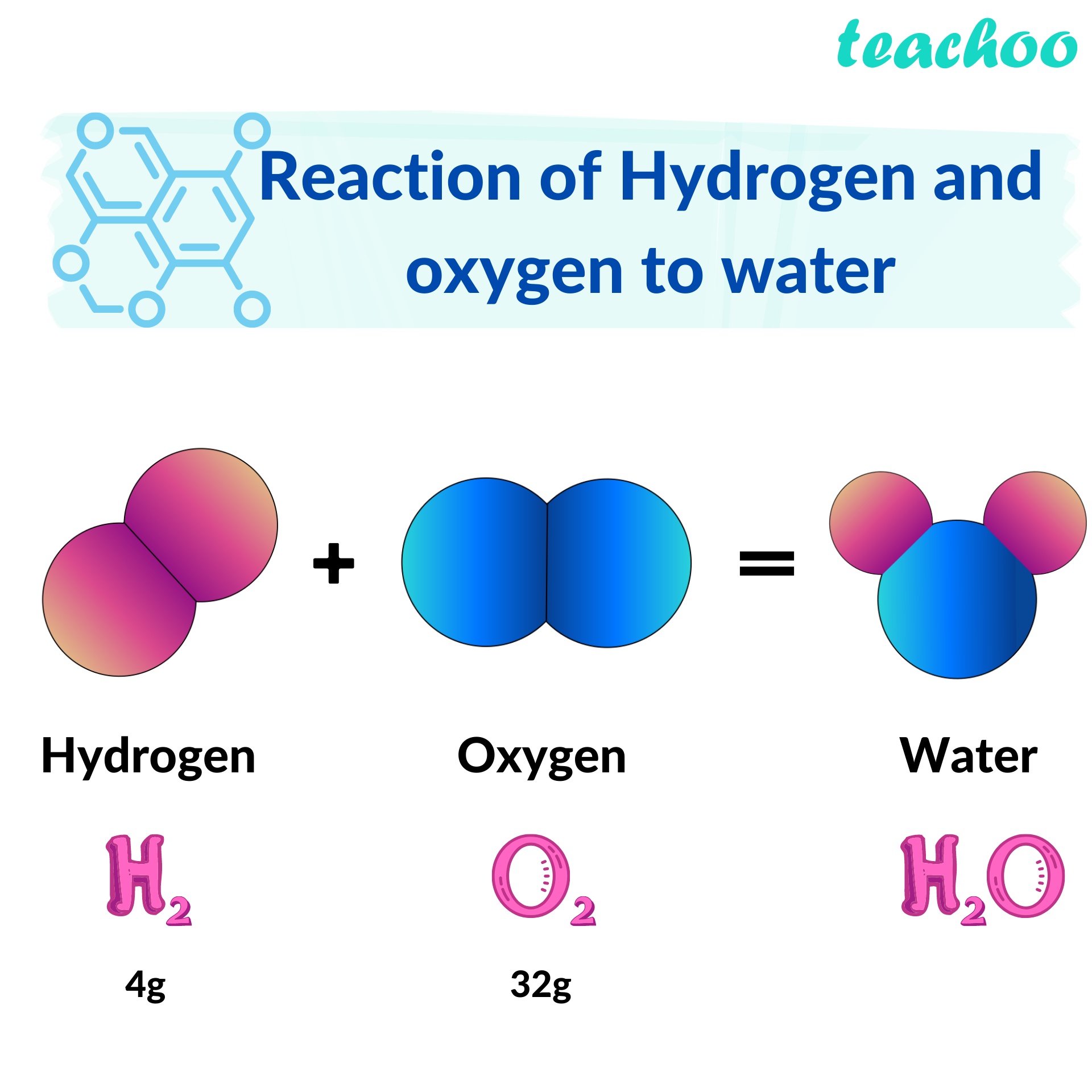
2. Hydrogen and oxygen combine in the ratio of 18 by mass to form water...
While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to.
Hydrogen and oxygen combine in the ratio of 18 by mass to form water
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively.
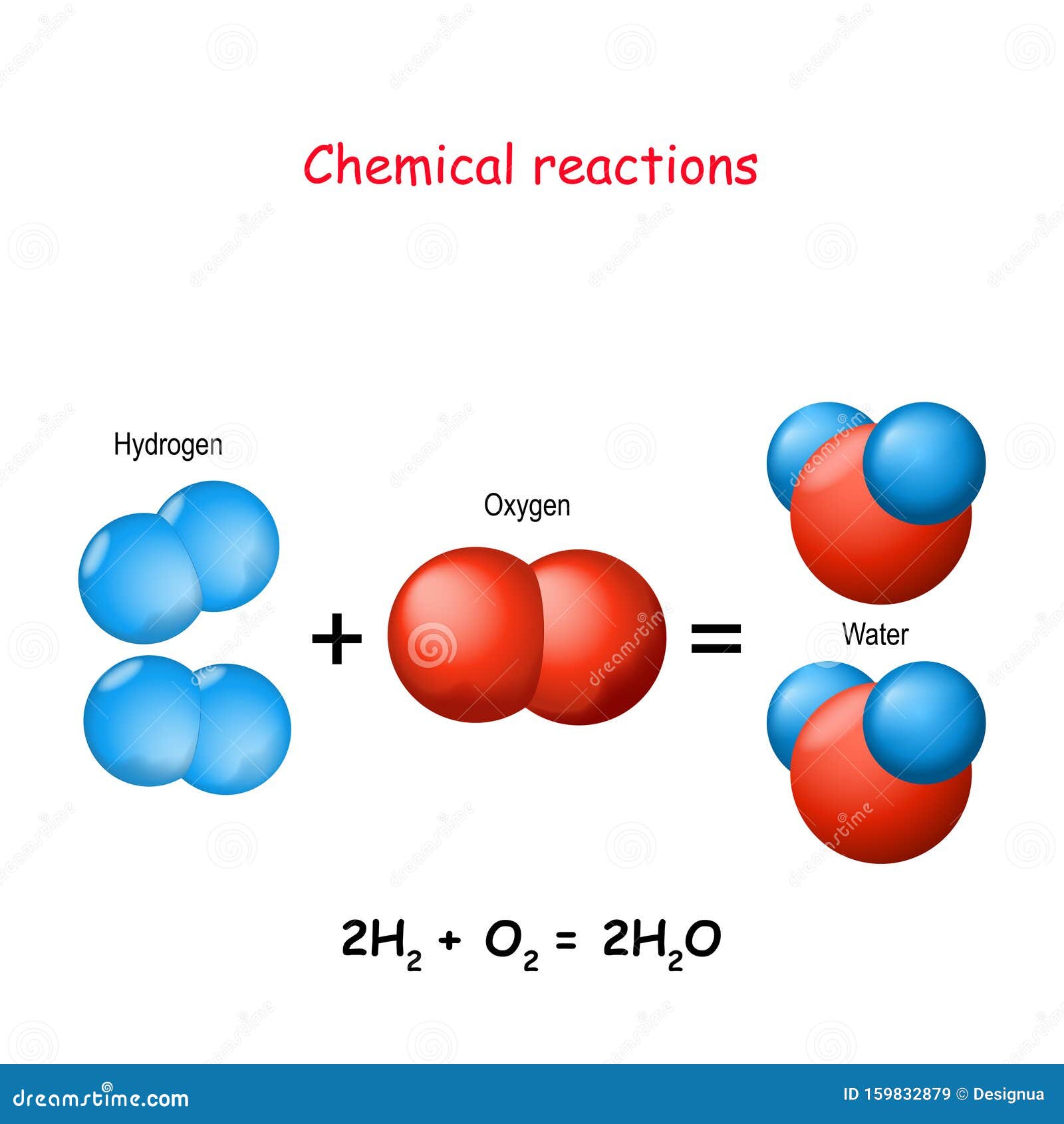
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed.
Compounds Water
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged.
Atomic Design Methodology Atomic Design by Brad Frost
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of.
[Solved] When hydrogen and oxygen combine to form water, water is
You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed.
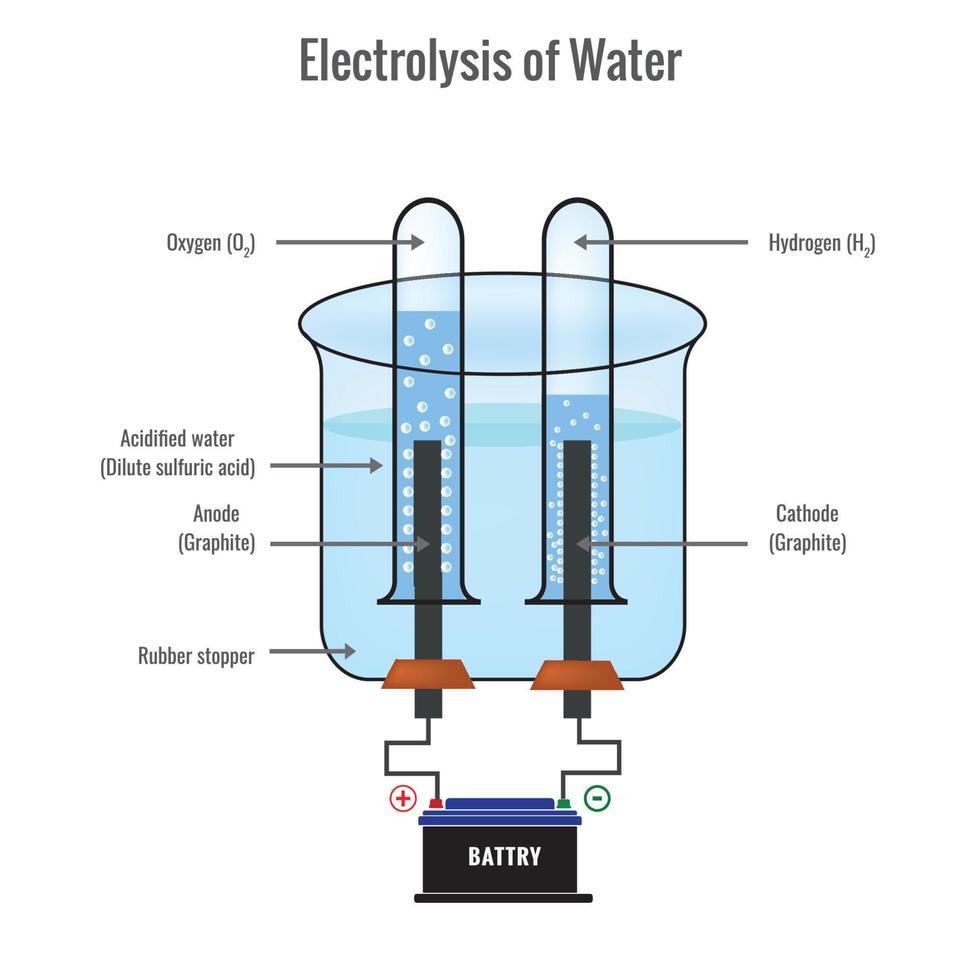
Electrolysis of water forming Hydrogen and Oxygen vector illustration
While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. You would need to combine two moles of hydrogen gas and one mole of oxygen gas to turn them into water. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged.
You Would Need To Combine Two Moles Of Hydrogen Gas And One Mole Of Oxygen Gas To Turn Them Into Water.
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen. While water can be formed through countless chemical reactions, the most efficient way to create a water molecule out of oxygen and. Hydrogen and oxygen atoms are attracted to one another by the electrostatic force between their positively charged protons and negatively charged.