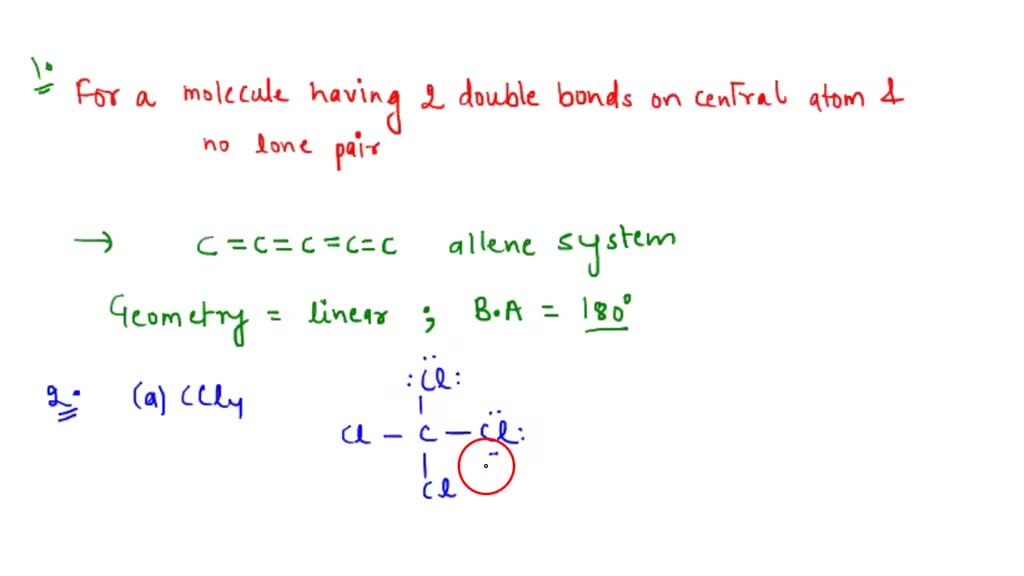
What Is The Value Of The Bond Angles In Sicl4 - What is the value of the bond angles in sicl_(4) ? Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: What is the value of the bond angles in sicl_(4) ? Sicl4 molecular and electron geometry. The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule.
Sicl4 molecular and electron geometry. What is the value of the bond angles in sicl_(4) ? What is the value of the bond angles in sicl_(4) ? The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule.
The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. Sicl4 molecular and electron geometry. What is the value of the bond angles in sicl_(4) ? What is the value of the bond angles in sicl_(4) ? The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule.
Scl4 Lewis Structure Molecular Geometry
What is the value of the bond angles in sicl_(4) ? Sicl4 molecular and electron geometry. The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. The bond angle of sicl4 is 109.5° as the shape of.
SOLVED The molecules SiCl4 and PCl3 have the same electrondomain
The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: What is the value of the bond angles in sicl_(4) ? What is the value of the bond angles.
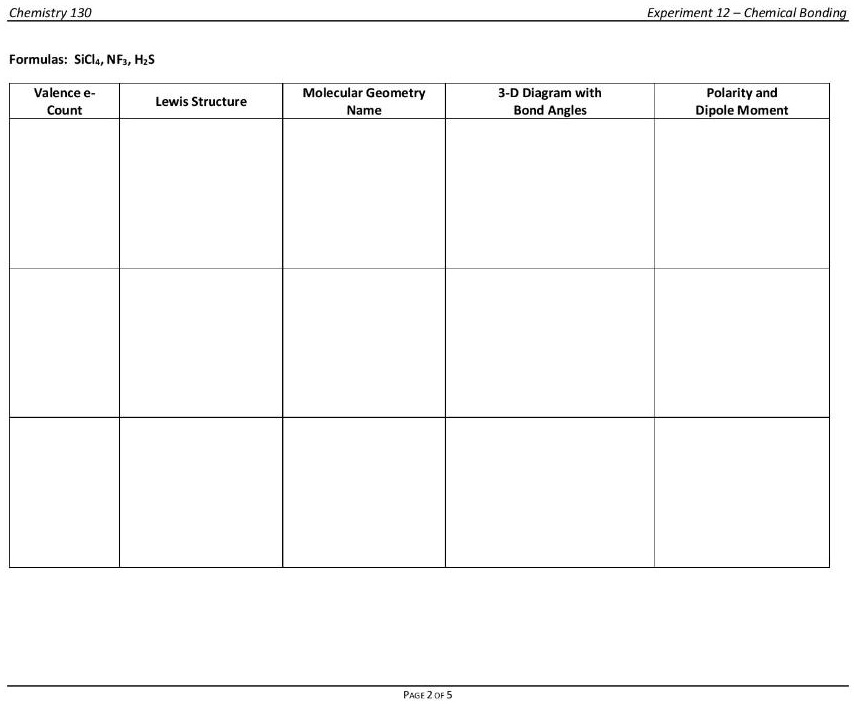
SOLVED Chemistry 130 Experiment 12 Chemical Bonding Formulas SiCl4
The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. What is the value of the bond angles in sicl_(4) ? What is the value of the bond angles in sicl_(4) ? Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond.
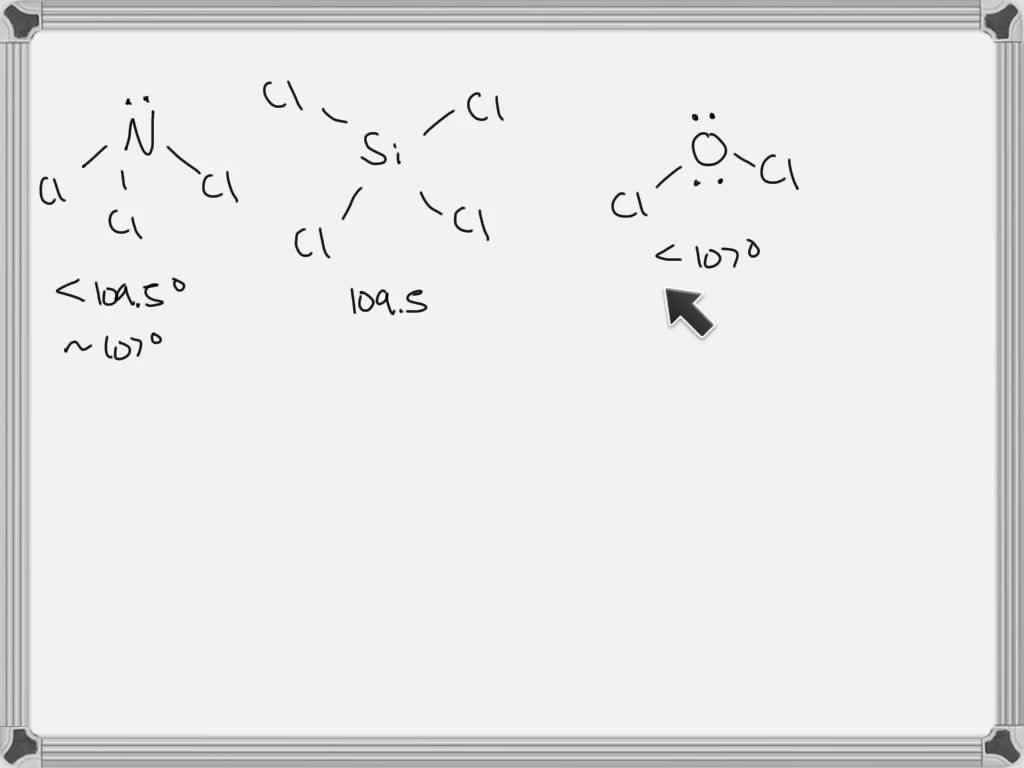
SOLVED The correct order of increasing ClXCl bond angles is I. NCl3
What is the value of the bond angles in sicl_(4) ? Sicl4 molecular and electron geometry. What is the value of the bond angles in sicl_(4) ? The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees.
SOLVEDFor each of the following species, write the Lewis structure
Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. What is the value of the bond angles in sicl_(4) ? What is the value of the bond angles in sicl_(4) ? The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: Sicl4 molecular and electron geometry.
predict the approximate bond angle in the following molecule.
Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. What is the value of the bond angles in sicl_(4) ? The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. What is the value of the bond.
Solved What is the value of the bond angles in SiCl4 ? Enter
The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. What is the value of the bond angles in sicl_(4) ? Part a) for sicl4, it has a tetrahedral.
Diagram of Bond Angles Quizlet
Sicl4 molecular and electron geometry. The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: What is the value of the bond angles in sicl_(4) ? The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. Part a) for.
Solved d. Use data about solid SiCl4 to estimate a value for
The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. What is the value of the bond angles in sicl_(4) ? Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees. What is the value of the bond.
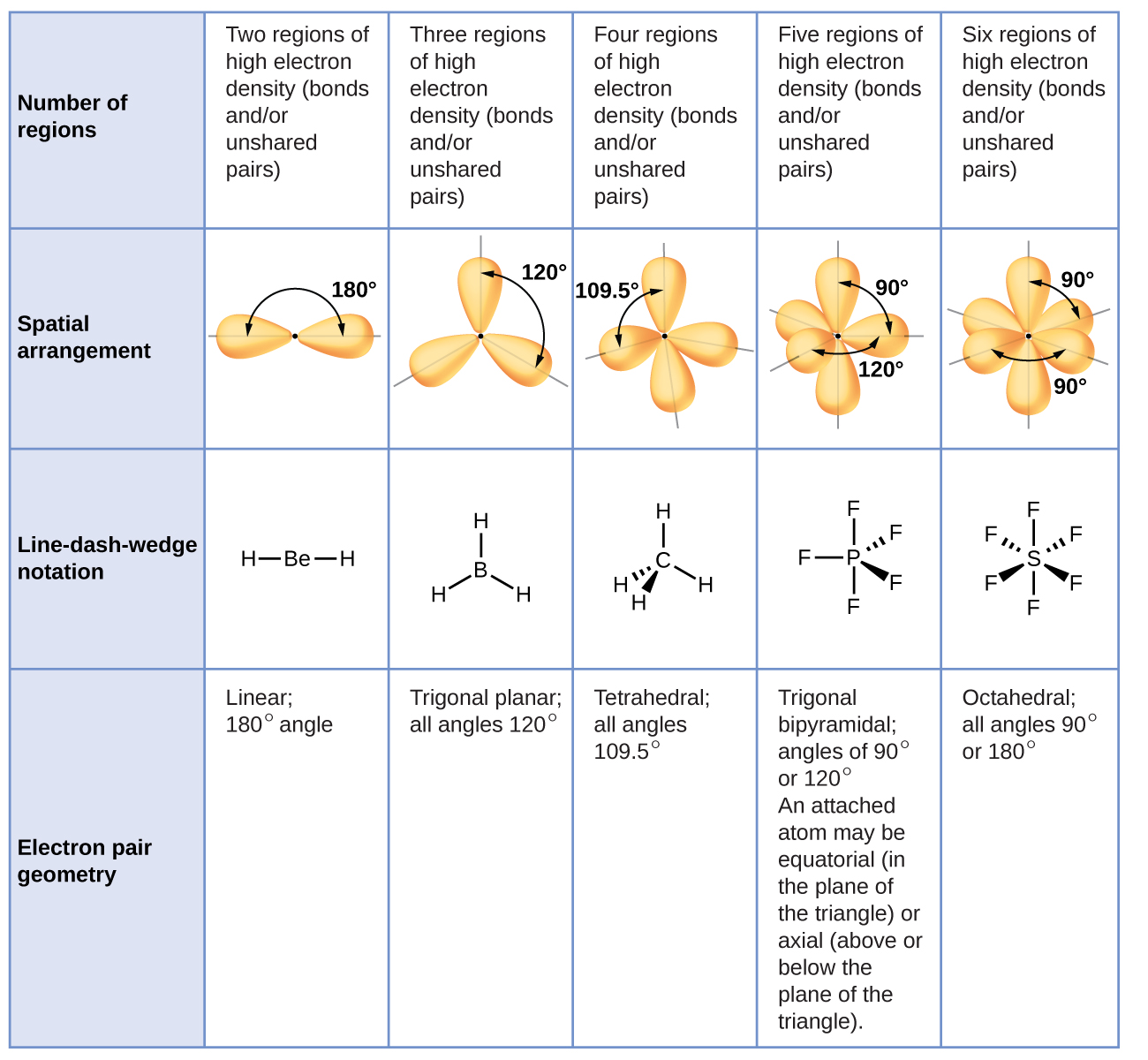
29+ how to calculate bond angles FerdavsAlaa
What is the value of the bond angles in sicl_(4) ? The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: The bond angle of sicl4 is 109.5° as the shape of its tetrahedral in nature and as per the vsepr theory, a regular tetrahedral molecule. Part a) for sicl4, it has a tetrahedral.
The Bond Angle Of Sicl4 Is 109.5° As The Shape Of Its Tetrahedral In Nature And As Per The Vsepr Theory, A Regular Tetrahedral Molecule.
What is the value of the bond angles in sicl_(4) ? The structure of s i c l x 4 \ce{sicl4} sicl x 4 is given below: What is the value of the bond angles in sicl_(4) ? Part a) for sicl4, it has a tetrahedral molecular geometry, therefore the bond angles are 109.5 degrees.