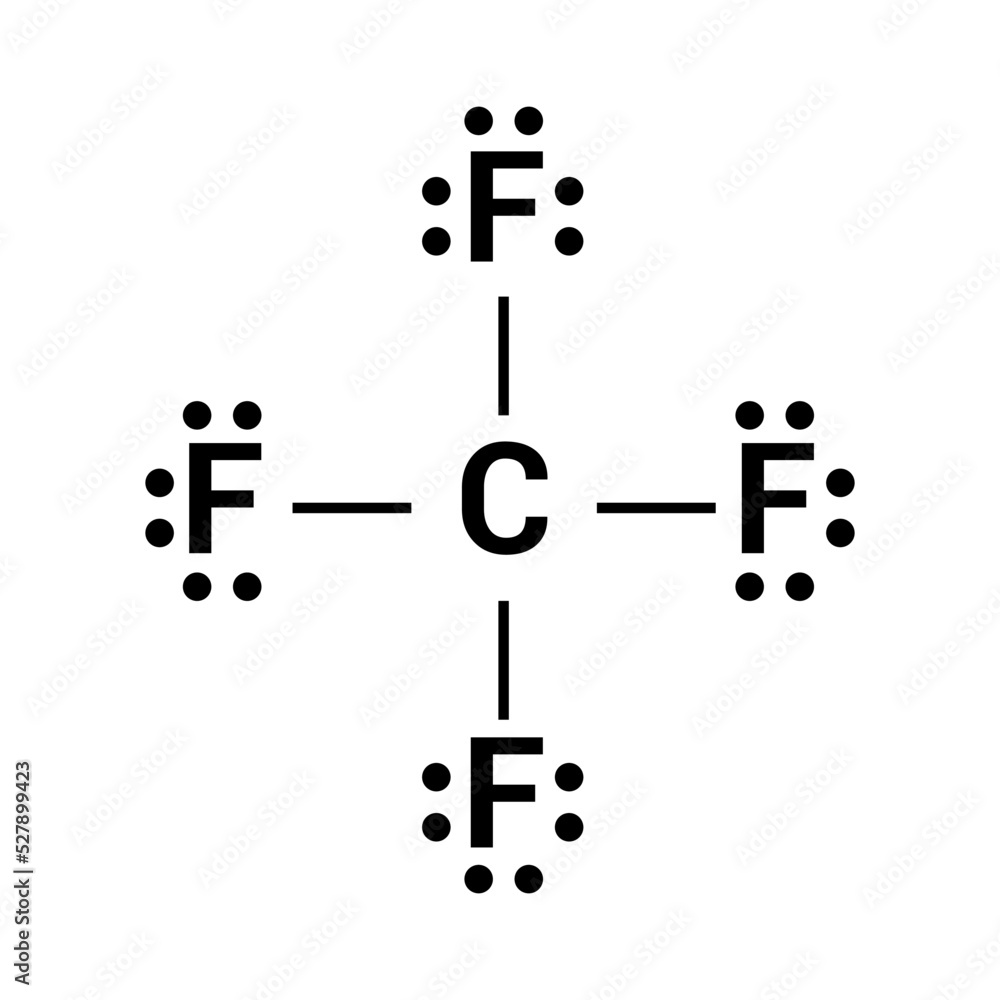
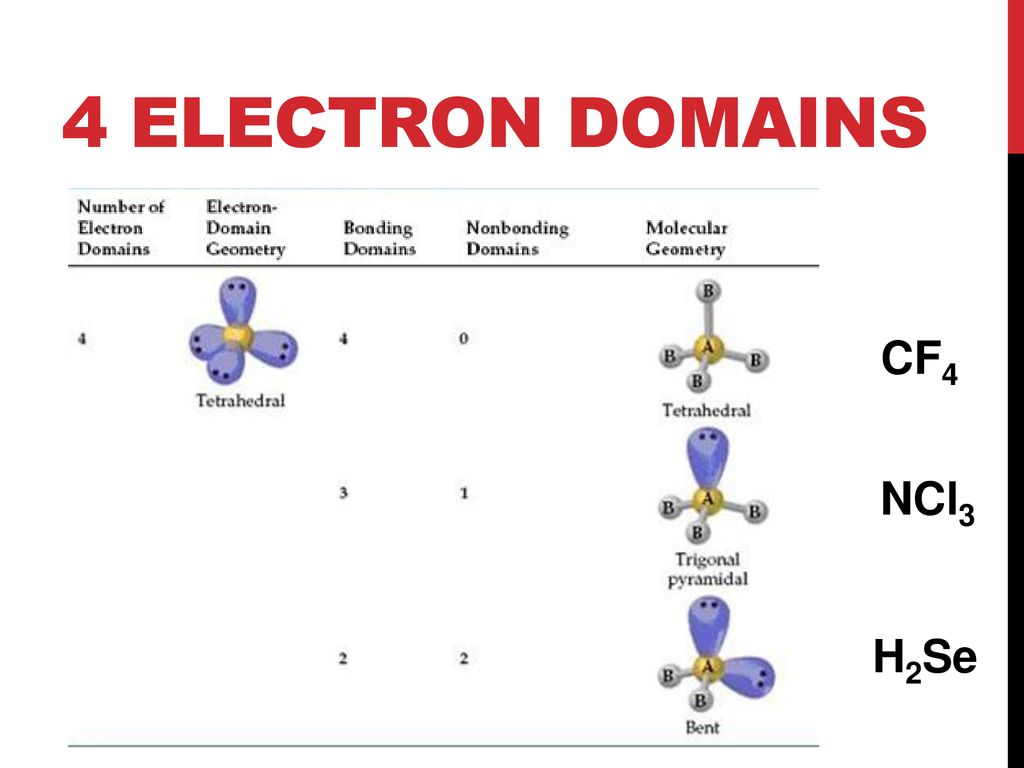
What Is The Molecular Geometry Of Cf4 - Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. The bond angle of cf4 is 109.5º.
Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. The bond angle of cf4 is 109.5º.
Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.
Lewis structure of carbon tetrafluoride (CF4) Stock Vector Adobe Stock
Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. The bond angle of cf4 is 109.5º. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom.
Pin on Geometry Of Molecules
The bond angle of cf4 is 109.5º. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.
Cf4 molecular geometry studentxoler
The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom.
Cf4 Molecular Geometry
Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. The bond angle of cf4 is 109.5º. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom.
CF4 (Carbon tetrafluoride) Molecular Geometry, Bond Angles & Electron
Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. The bond angle of cf4 is 109.5º.
Tetrafluoromethane Lewis structure Carbon tetrachloride Silicon
The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom.
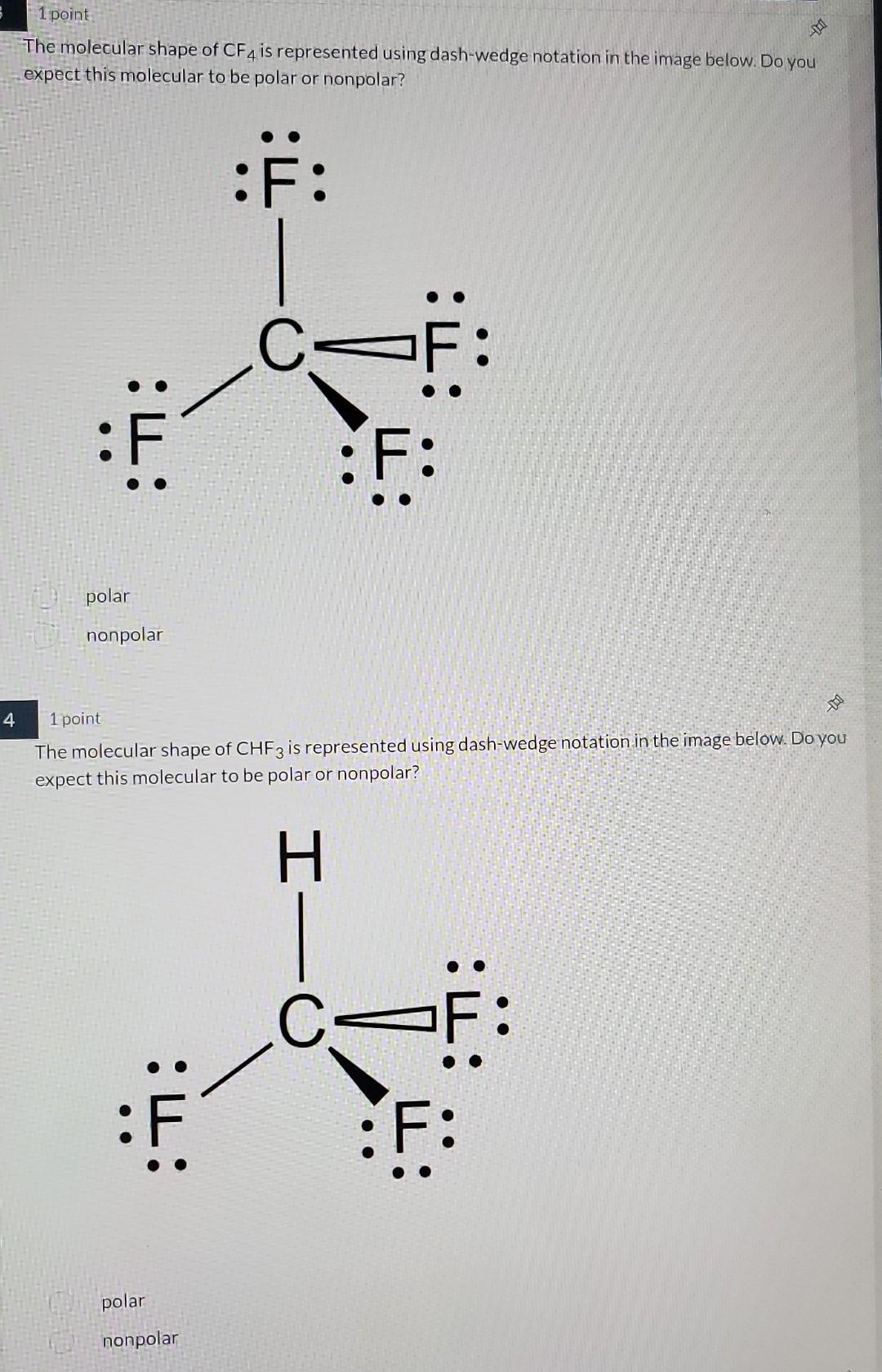
Polarity of Carbon Tetrafluoride (CF4)
The bond angle of cf4 is 109.5º. Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.
CF4 Molecular Geometry, Bond Angles & Electron Geometry (Carbon
Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.
Solved The molecular shape of CF4 is represented using
Cf4 has a tetrahedral geometry, where the four fluorine atoms are symmetrically arranged around the carbon atom. The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.
Cf4 Has A Tetrahedral Geometry, Where The Four Fluorine Atoms Are Symmetrically Arranged Around The Carbon Atom.
The bond angle of cf4 is 109.5º. Cf4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral.