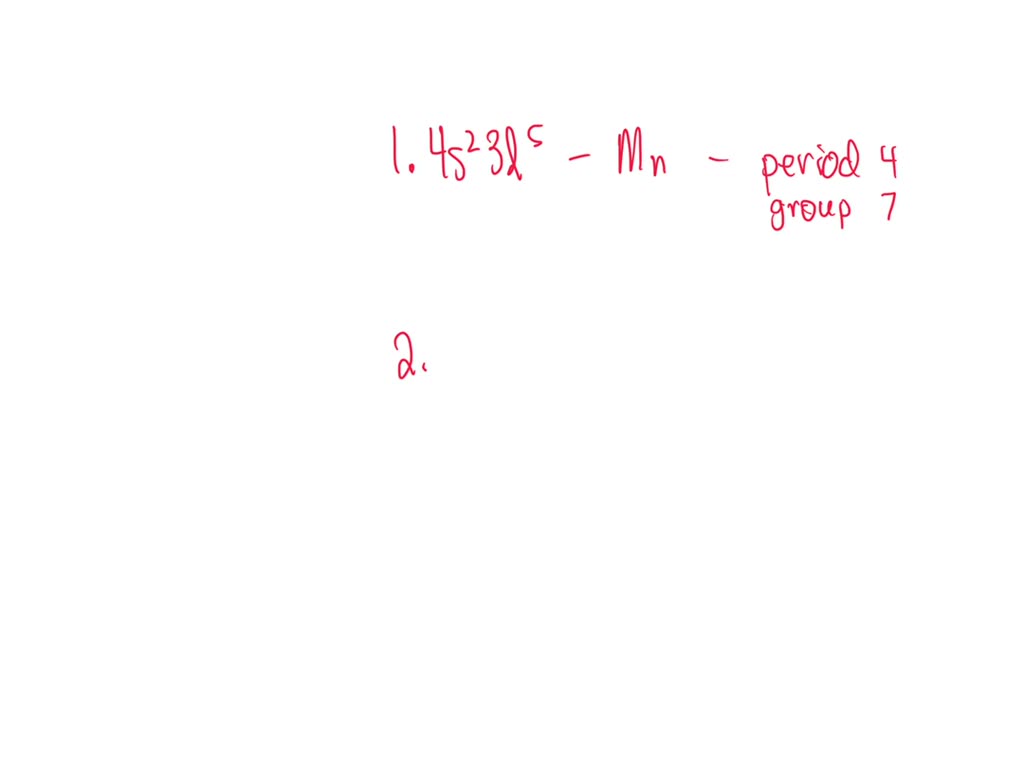What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D5 - The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. 119 rows the electron configuration shows the distribution of electrons into subshells.
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. 119 rows the electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn).
119 rows the electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the.
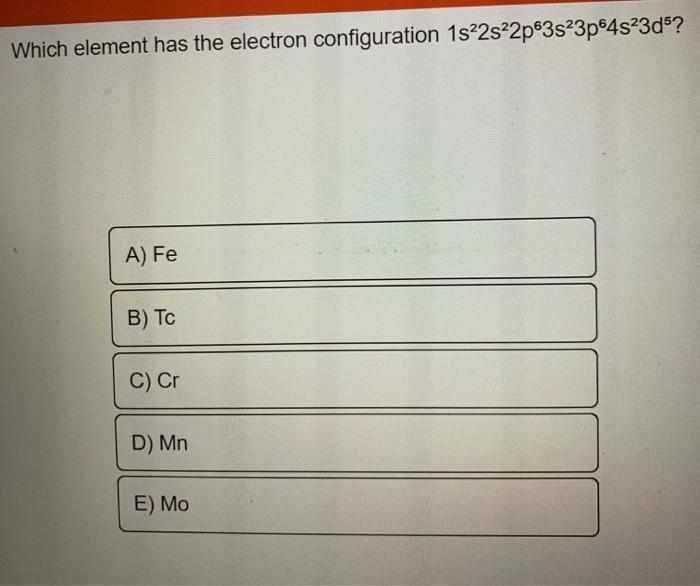
[Solved] Which element has the following electron configuration
This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). Study with quizlet and memorize flashcards.
Electronic Configuration Antimony Learn Important Terms and Concepts
Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. This list of electron configurations of elements. 119 rows the electron configuration shows the distribution of electrons into subshells. The electron configuration 1s22s22p63s23p64s23d5.
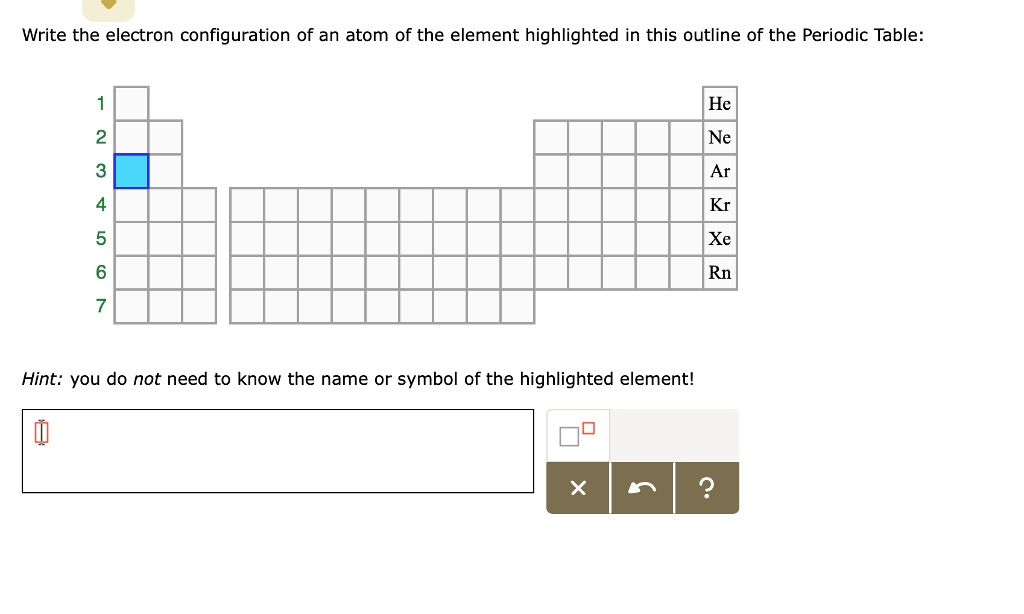
write the electron configuration of an atom of the element highlighted
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. 119 rows the electron configuration shows the distribution of electrons into subshells. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese.
Electron Configuration of Elements Chemistry Periodic Table
Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. 119.
Solved Which element has the electron configuration
Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. This list of electron configurations of elements. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). 119 rows the electron configuration shows.
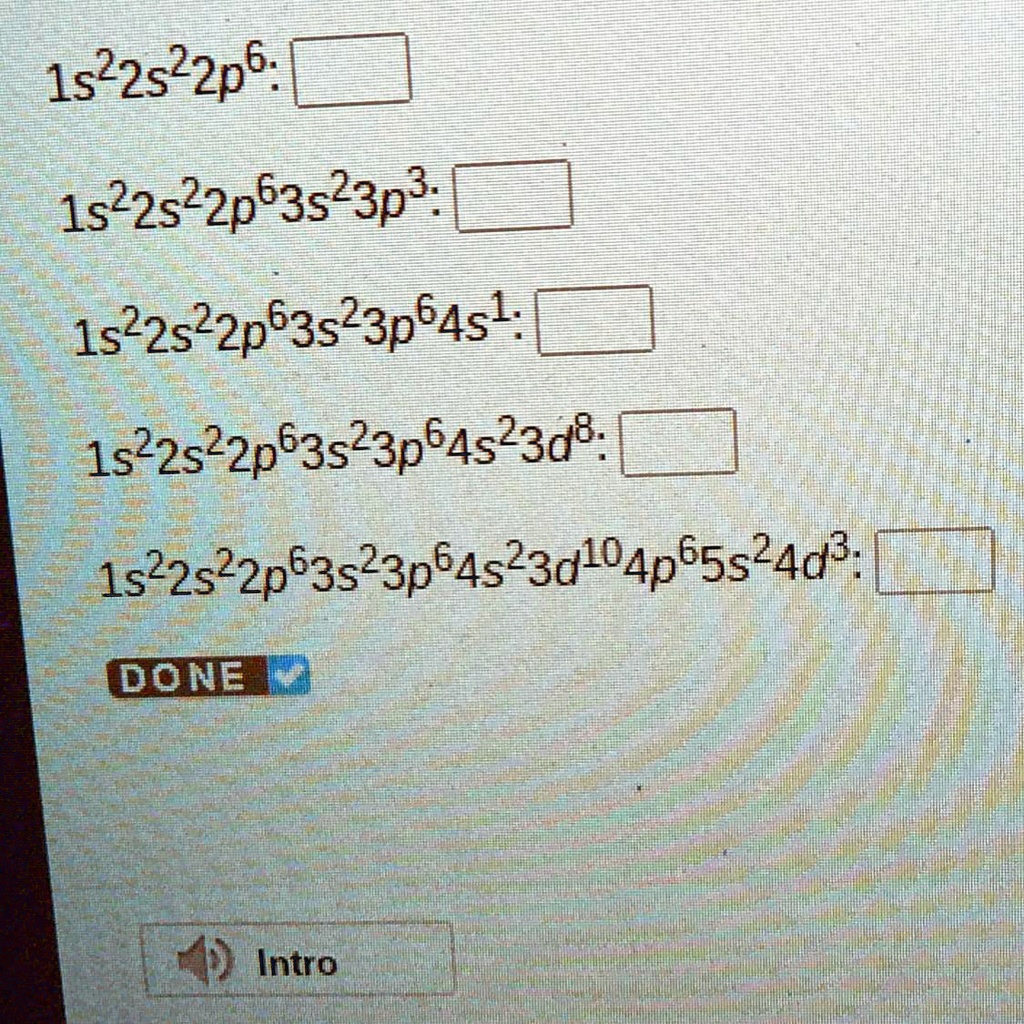
1.5 Electronic Structure of Atoms (Electron Configurations)
Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. This.
use the periodic table to identify the element indicated by each
119 rows the electron configuration shows the distribution of electrons into subshells. Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for k+?, elements that have similar. To identify the element with the.
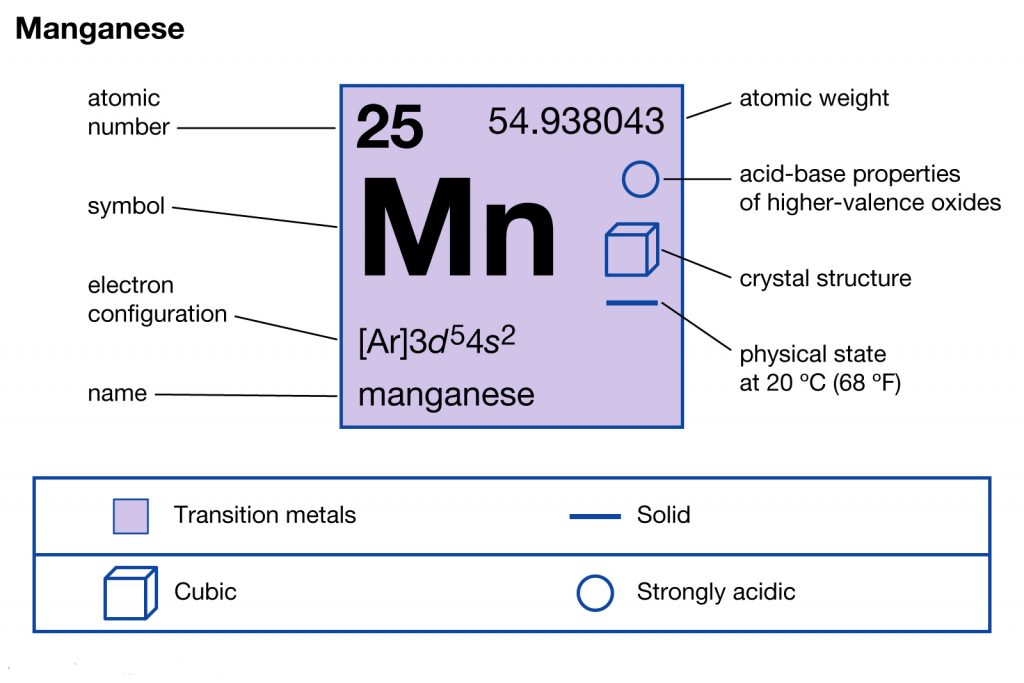
Manganese Electron Configuration (Mn) with Orbital Diagram
119 rows the electron configuration shows the distribution of electrons into subshells. To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. This list of electron configurations of elements. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. Study with.
(1) The element with an electron configuration of 1s22s22p63s23p64s23d5
Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an. 119 rows the electron configuration shows the distribution of electrons into subshells. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. To identify the element with the electron configuration ,.
Electron configuration of every element in the periodic table
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. 119 rows the electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements. The electron.
Study With Quizlet And Memorize Flashcards Containing Terms Like What Is The Electron Configuration For K+?, Elements That Have Similar.
To identify the element with the electron configuration , first count the total number of electrons by adding the exponents of the. The element with the electron configuration 1s22s22p63s23p64s23d5 is vanadium (v), which has an atomic number of 23. The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (mn). Study with quizlet and memorize flashcards containing terms like which sequence of elements is arranged in order of decreasing atomic radii?, an.
119 Rows The Electron Configuration Shows The Distribution Of Electrons Into Subshells.
This list of electron configurations of elements.