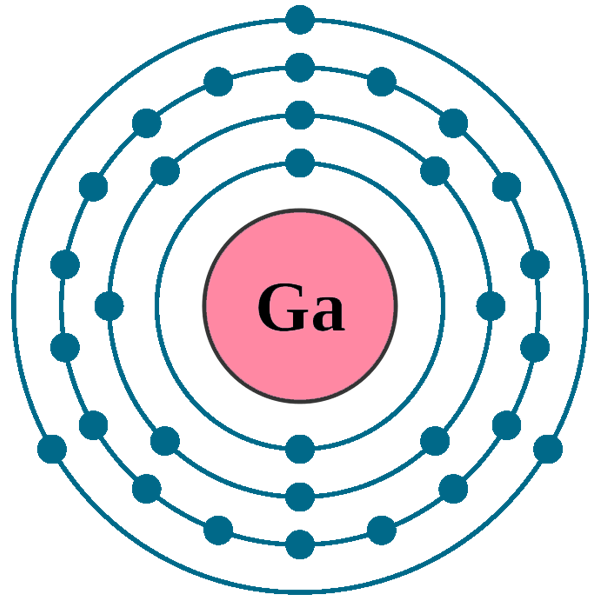
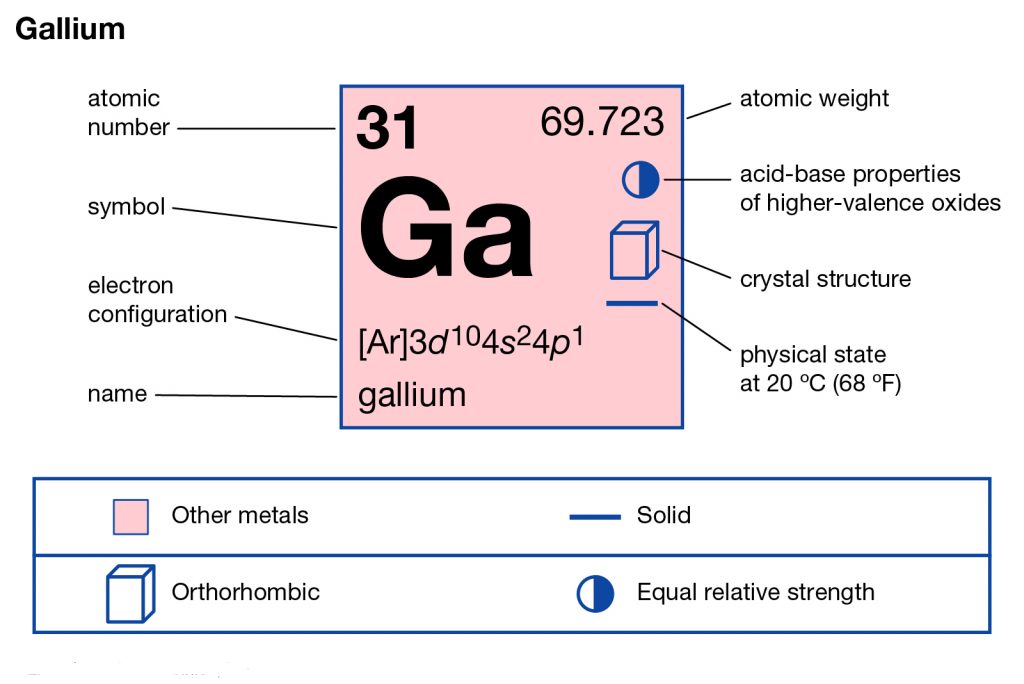
What Is The Electron Configuration Of Gallium - Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra.
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
Gallium Ga (Element 31) of Periodic Table Element FlashCards
Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found.
Gallium Element Facts
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
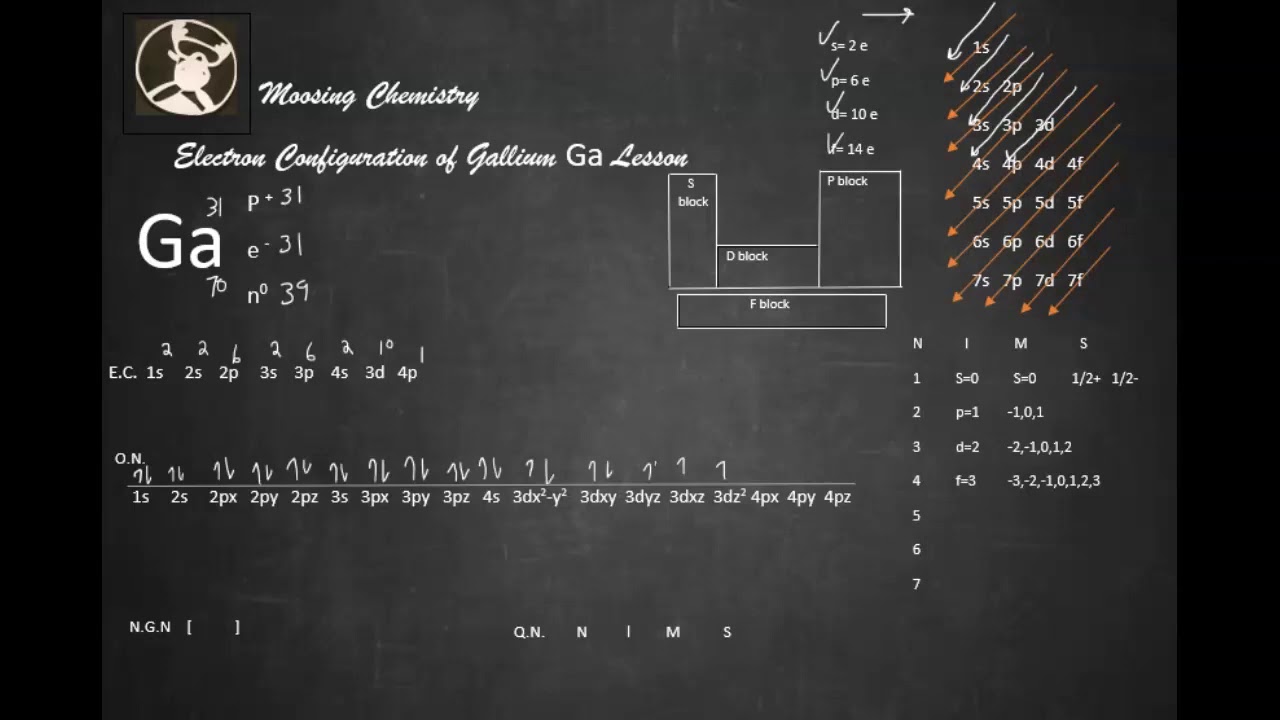
Electron Configuration for Gallium (Ga, Ga3+ ion)
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
Gallium Lewis Dot Structure
These blocks are named for the characteristic spectra. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. Elements are organised into blocks by the orbital type in which the outer electrons are found.
Ga electronic configurationHow to write electronic configuration of
Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. These blocks are named for the characteristic spectra.
Electron Configuration Of Gallium
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
A stepbystep description of how to write the electron configuration
These blocks are named for the characteristic spectra. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. Elements are organised into blocks by the orbital type in which the outer electrons are found.
Electron Configuration For Gallium Archives Dynamic Periodic Table of
Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. These blocks are named for the characteristic spectra.
Solved The electron configuration of the gallium (III) ion
Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra.
Gallium Lewis Dot Structure
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as.
These Blocks Are Named For The Characteristic Spectra.
Gallium is a chemical element of the periodic table with chemical symbol ga and atomic number 31 with an atomic weight of 69.7231 u and is classed as. Elements are organised into blocks by the orbital type in which the outer electrons are found.