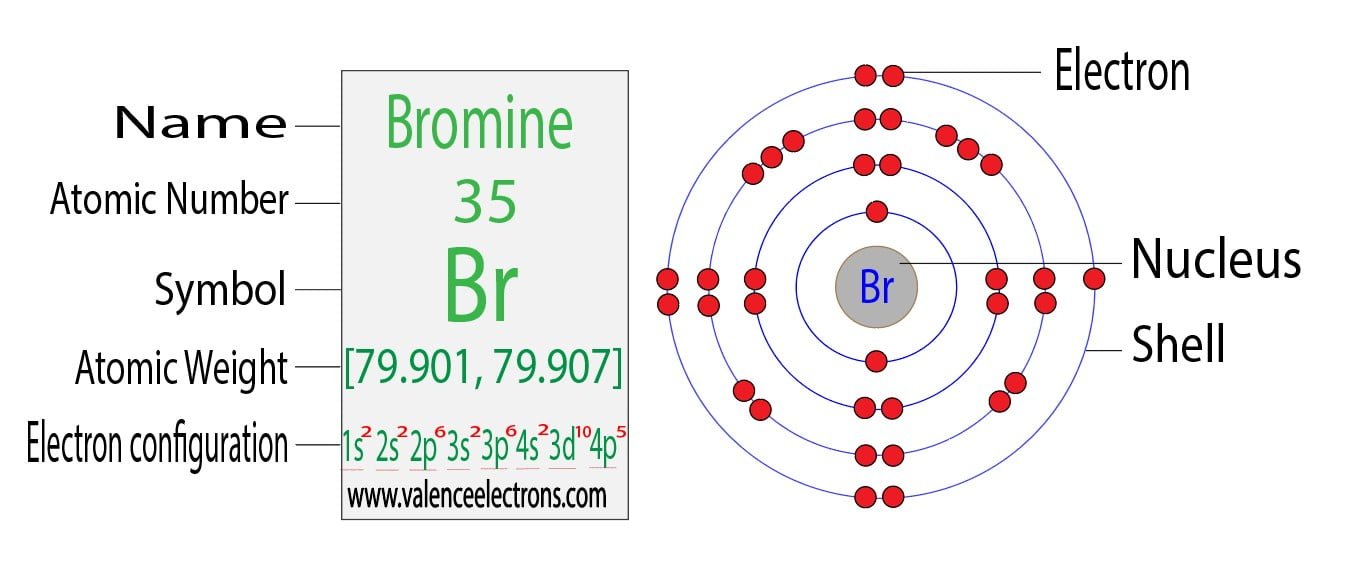
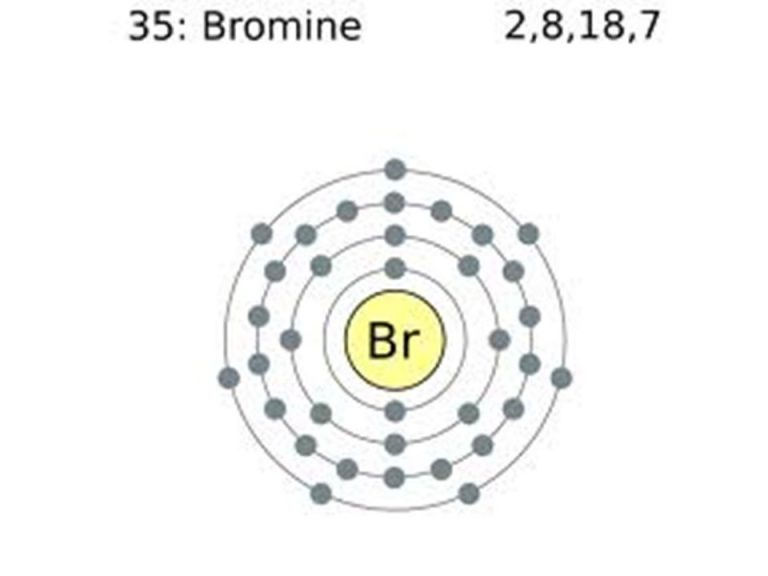
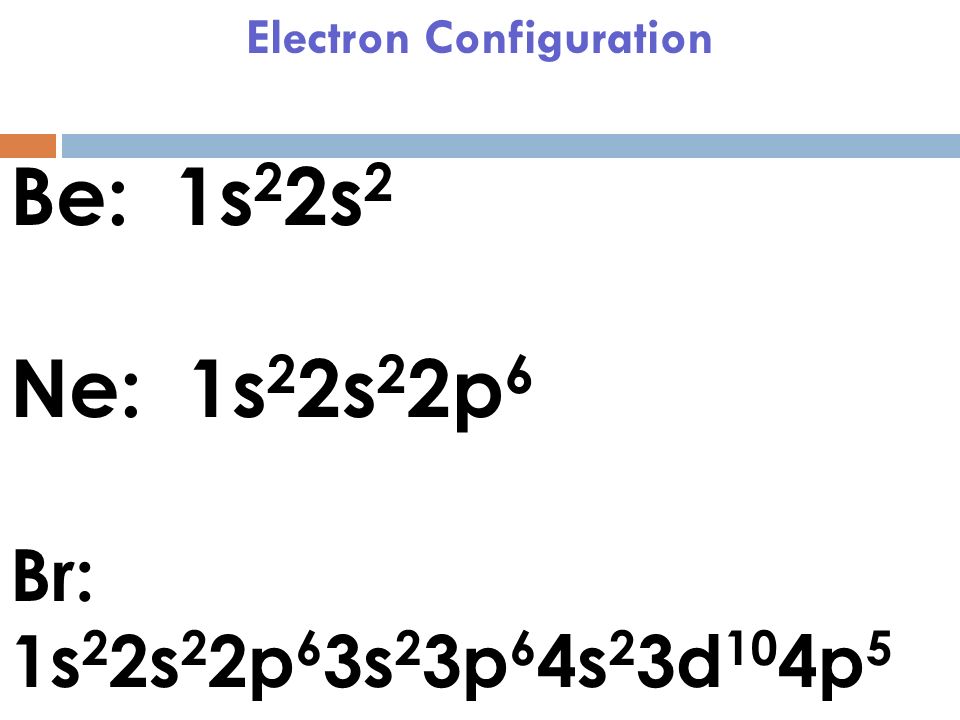
What Is The Electron Configuration Of Br - Bromine belongs to group 17 which is known as halogens. If it shows unpaired electrons, then the substance. Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra. Atomic mass, electron configurations, charges, and more. The magnetic form of a substance can be determined by examining its electron configuration: View rotating bohr models for all 118. Access detailed info on all elements:
The magnetic form of a substance can be determined by examining its electron configuration: Elements are organised into blocks by the orbital type in which the outer electrons are found. If it shows unpaired electrons, then the substance. Access detailed info on all elements: View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more.
These blocks are named for the characteristic spectra. View rotating bohr models for all 118. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. If it shows unpaired electrons, then the substance. The magnetic form of a substance can be determined by examining its electron configuration: Access detailed info on all elements:
Br Electron Configuration (Bromide Ion) YouTube
Access detailed info on all elements: The magnetic form of a substance can be determined by examining its electron configuration: View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens.
Atom Diagrams Electron Configurations of the Elements
Access detailed info on all elements: These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. The magnetic form of a substance can be determined by examining its electron configuration:
Electron Configuration For Bromine
Bromine belongs to group 17 which is known as halogens. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Access detailed info on all elements: Atomic mass, electron configurations, charges, and more.
Electron Configuration Magnesium
The magnetic form of a substance can be determined by examining its electron configuration: Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. These blocks are named for the characteristic spectra. View rotating bohr models for all 118.
Bromine Electron Configuration (Br) with Orbital Diagram
Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. Elements are organised into blocks by the orbital type in which the outer electrons are found. The magnetic form of a substance can be determined by examining its electron configuration:
Electron Configuration For Bromine
These blocks are named for the characteristic spectra. The magnetic form of a substance can be determined by examining its electron configuration: Atomic mass, electron configurations, charges, and more. Elements are organised into blocks by the orbital type in which the outer electrons are found. View rotating bohr models for all 118.
Electronic Configurations Intro Chemistry LibreTexts
These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118.
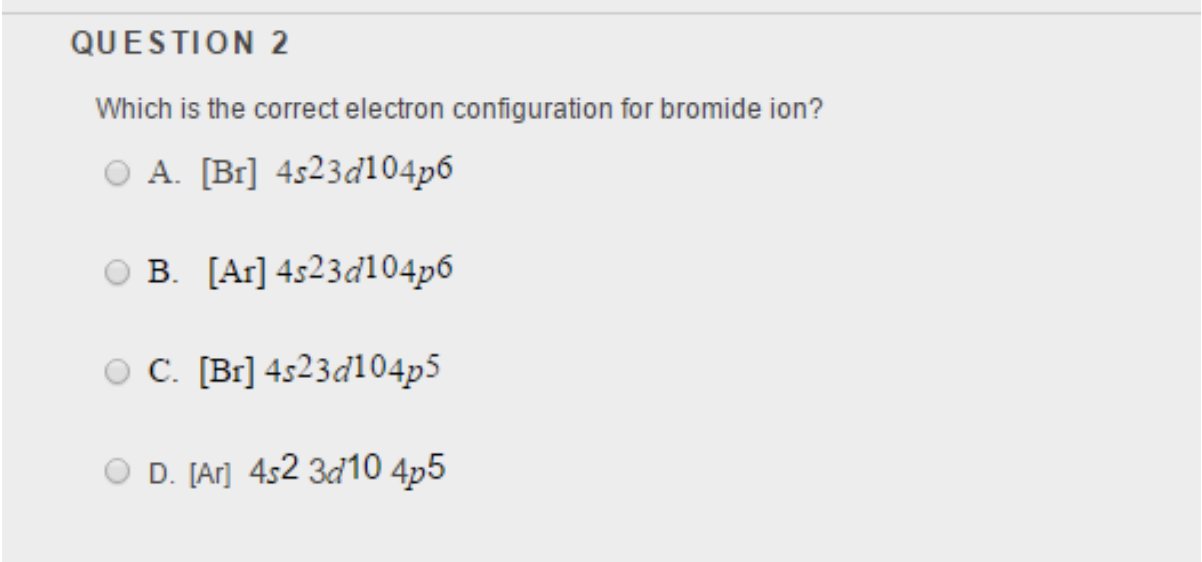
Solved QUESTION 2 Which is the correct electron
Access detailed info on all elements: These blocks are named for the characteristic spectra. Atomic mass, electron configurations, charges, and more. If it shows unpaired electrons, then the substance. View rotating bohr models for all 118.
Cr2+ Ground State Electron Configuration Electron Configurations
Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. View rotating bohr models for all 118. If it shows unpaired electrons, then the substance. Access detailed info on all elements:
Bromine Belongs To Group 17 Which Is Known As Halogens.
View rotating bohr models for all 118. Access detailed info on all elements: Atomic mass, electron configurations, charges, and more. If it shows unpaired electrons, then the substance.
Elements Are Organised Into Blocks By The Orbital Type In Which The Outer Electrons Are Found.
These blocks are named for the characteristic spectra. The magnetic form of a substance can be determined by examining its electron configuration:
:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)