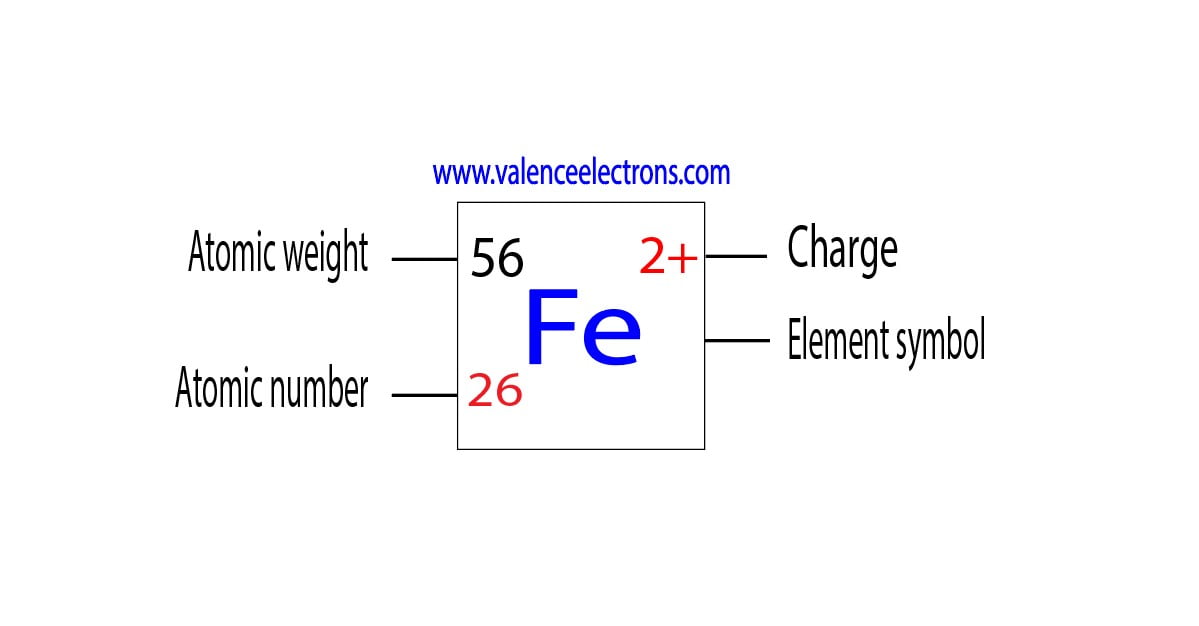
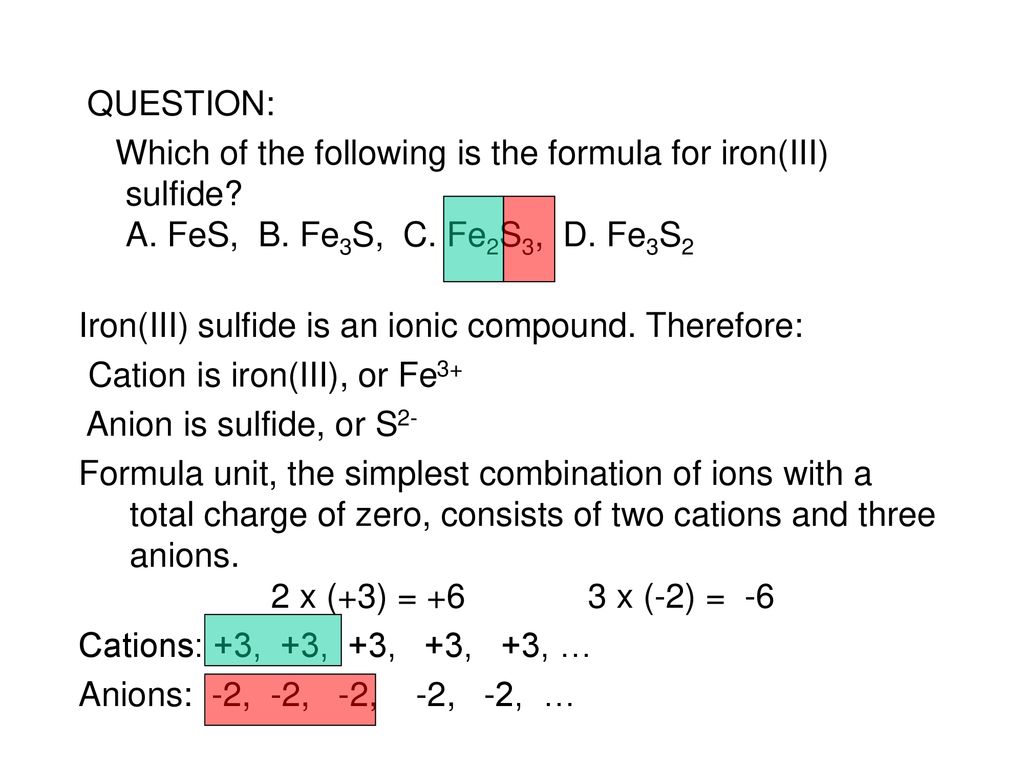
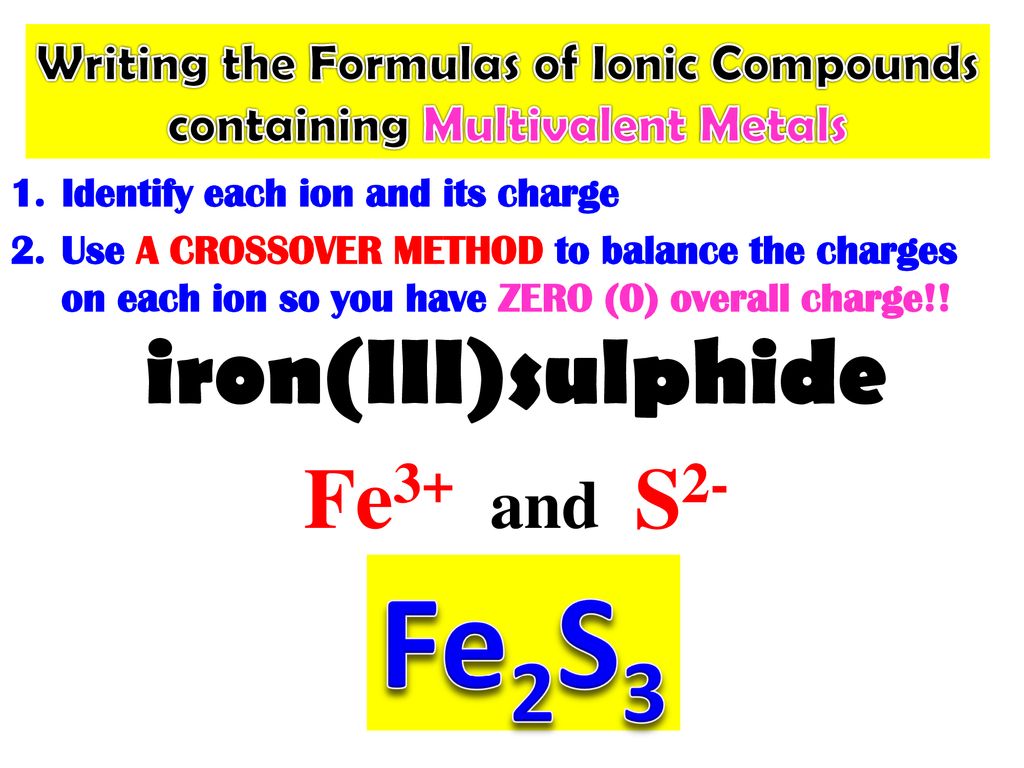
What Is The Charge On The Iron Ion In Fe2S3 - In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. What is the charge on the iron in fe2s3? Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. In an ionic molecule the formula is. Essentially, the formula fe2s3 indicates that the. It is a solid, black powder that. The correct formula for iron(iii) sulfide is fe2s3. Your solution’s ready to go!
The correct formula for iron(iii) sulfide is fe2s3. Your solution’s ready to go! Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. What is the charge on the iron in fe2s3? In an ionic molecule the formula is. Essentially, the formula fe2s3 indicates that the. It is a solid, black powder that. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons.
Essentially, the formula fe2s3 indicates that the. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. The correct formula for iron(iii) sulfide is fe2s3. Your solution’s ready to go! Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. It is a solid, black powder that. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. What is the charge on the iron in fe2s3? In an ionic molecule the formula is. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons.
What Is the Charge on the Iron Ion in Fe2s3
In an ionic molecule the formula is. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. The correct formula for iron(iii) sulfide is fe2s3. It is a.
How to Write the Net Ionic Equation for Fe2S3 + HBr = H2S + FeBr3 YouTube
Essentially, the formula fe2s3 indicates that the. In an ionic molecule the formula is. Your solution’s ready to go! The correct formula for iron(iii) sulfide is fe2s3. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion.
Ionic Charge for Iron (Fe) YouTube
It is a solid, black powder that. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. What is the charge on the iron in fe2s3? In an ionic molecule the formula is. Your solution’s ready to go!
3.2 Names and Formulas of Ionic Compounds ppt download
In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Essentially, the formula fe2s3 indicates that the. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. What is the charge on the iron in fe2s3? Explanation the.
What Is Fe3 And Fe2? Quick Answer
Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Essentially, the formula fe2s3 indicates that the. The correct formula for iron(iii) sulfide is fe2s3. What is the.
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry
In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. What is the charge on the iron in fe2s3? Your solution’s ready to go! Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. The correct formula for.
What Is the Charge on the Iron Ion in Fe2s3
In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. In an ionic molecule the formula is. What is the charge on the iron in fe2s3? Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. Explanation the.
Nomenclature Part I PO43 phosphate ion HC2H3O2 Acetic acid C2H3O2
What is the charge on the iron in fe2s3? Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. It is a solid, black powder that. In the.
Question Video Deducing the Ionic Formula of an Ionic Compound Where
Your solution’s ready to go! Essentially, the formula fe2s3 indicates that the. The correct formula for iron(iii) sulfide is fe2s3. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. It is a solid, black powder that.
How Many Protons, Neutrons and Electrons Does Iron Have?
It is a solid, black powder that. Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. Your solution’s ready to go! Essentially, the formula fe2s3 indicates that the. The correct formula for iron(iii) sulfide is fe2s3.
It Is A Solid, Black Powder That.
Explanation the roman numeral (iii) in the name iron(iii) sulfide indicates the charge of the iron ion. What is the charge on the iron in fe2s3? Iron(iii) sulfide, also known as ferric sulfide or sesquisulfide (fe 2 s 3), is one of the several binary iron sulfides. In an ionic molecule the formula is.
Your Solution’s Ready To Go!
The correct formula for iron(iii) sulfide is fe2s3. In the ionic compound fes (iron sulfide), the iron ion has a charge of fe2+, meaning it has lost two electrons. Each ion of iron (iii) in fe 2 s 3 \text{fe}_2\text{s}_3 fe 2 s 3 is carrying a charge of 3+. Essentially, the formula fe2s3 indicates that the.





+iron+(III)+chloride.jpg)