What Is Conserved During A Chemical Reaction - The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the.
Solved Mass is conserved during any chemical reaction. What does this
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
Which diagram illustrates that matter is always conserved during a
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. For example, when wood burns, the. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
PPT Atoms and Mass are conserved during a chemical reaction
For example, when wood burns, the. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
Newsela The conservation of matter during physical and chemical changes
For example, when wood burns, the. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
Based on the diagram, which statement explains how energy is conserved
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
Question Video Identifying Which Statement Correctly Describes Mass
For example, when wood burns, the. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
Based on the diagram, which statement explains how energy is conserved
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. For example, when wood burns, the. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
Chemical reactions and enzymes ppt download
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. For example, when wood burns, the.
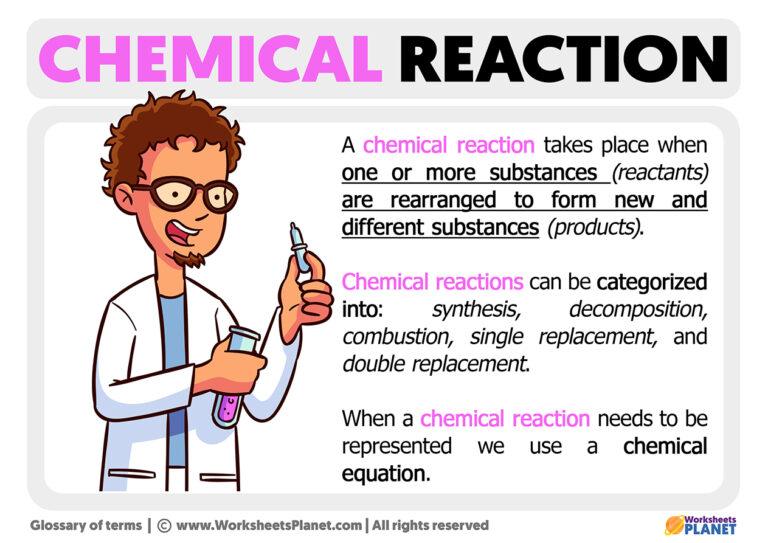
What is a Chemical Reaction?
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the.
Chapter 7 Chemical Reactions ppt download
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.
For Example, When Wood Burns, The.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants.