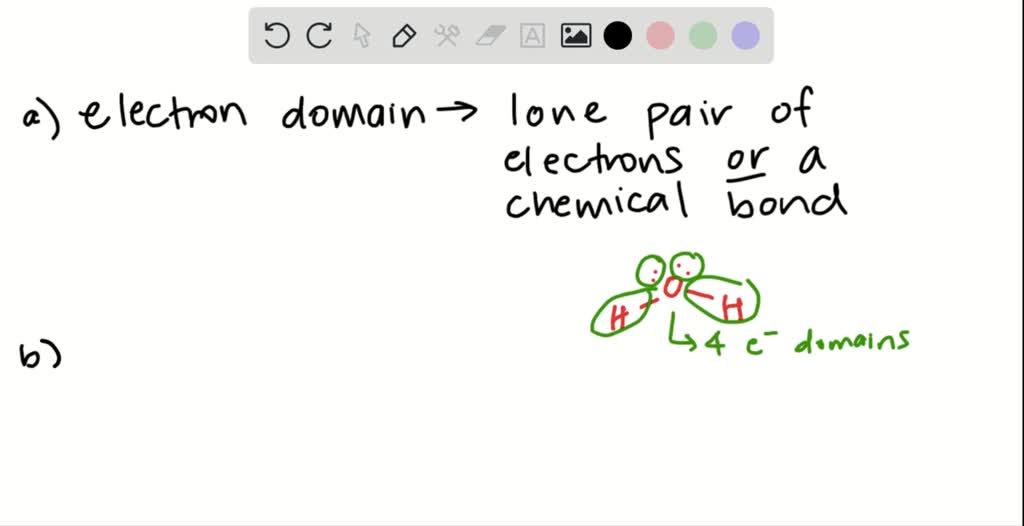
What Are Electron Domains - Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. It is a fundamental concept in the study of atomic. A lone pair, single, double, or triple. An electron domain is the region of space around an atom where one or more electrons are found.
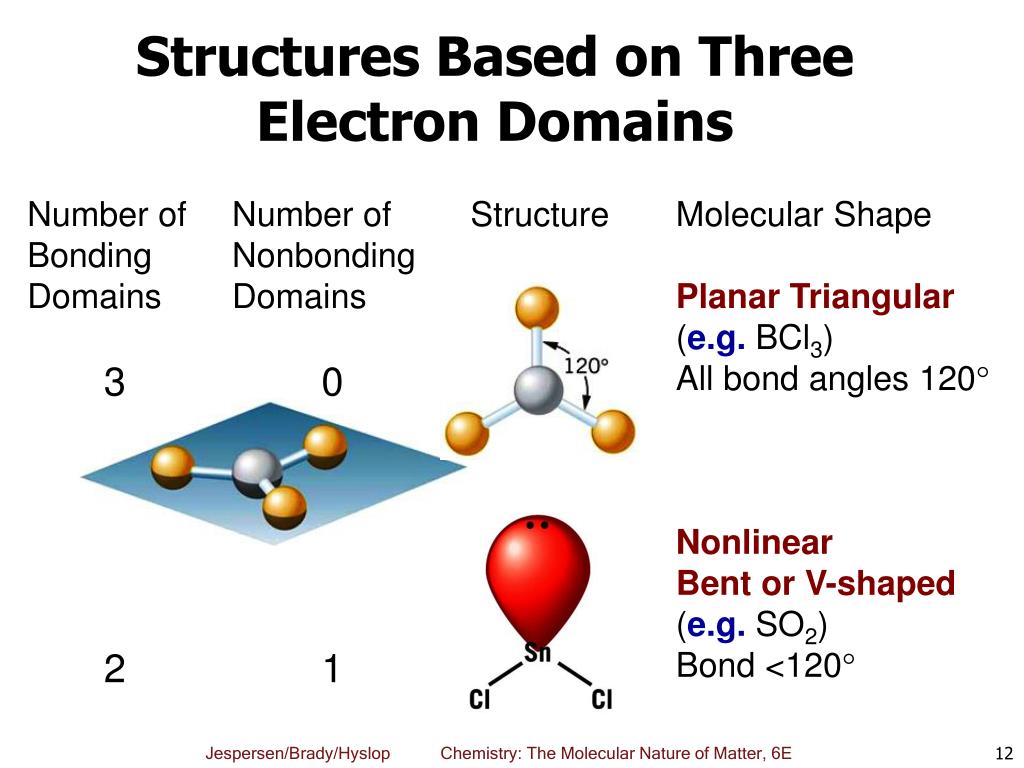
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. A lone pair, single, double, or triple. It is a fundamental concept in the study of atomic. An electron domain is the region of space around an atom where one or more electrons are found. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds.
A lone pair, single, double, or triple. An electron domain is the region of space around an atom where one or more electrons are found. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. It is a fundamental concept in the study of atomic.
PPT Chapter 10 Theories of Bonding and Structure PowerPoint
Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding.
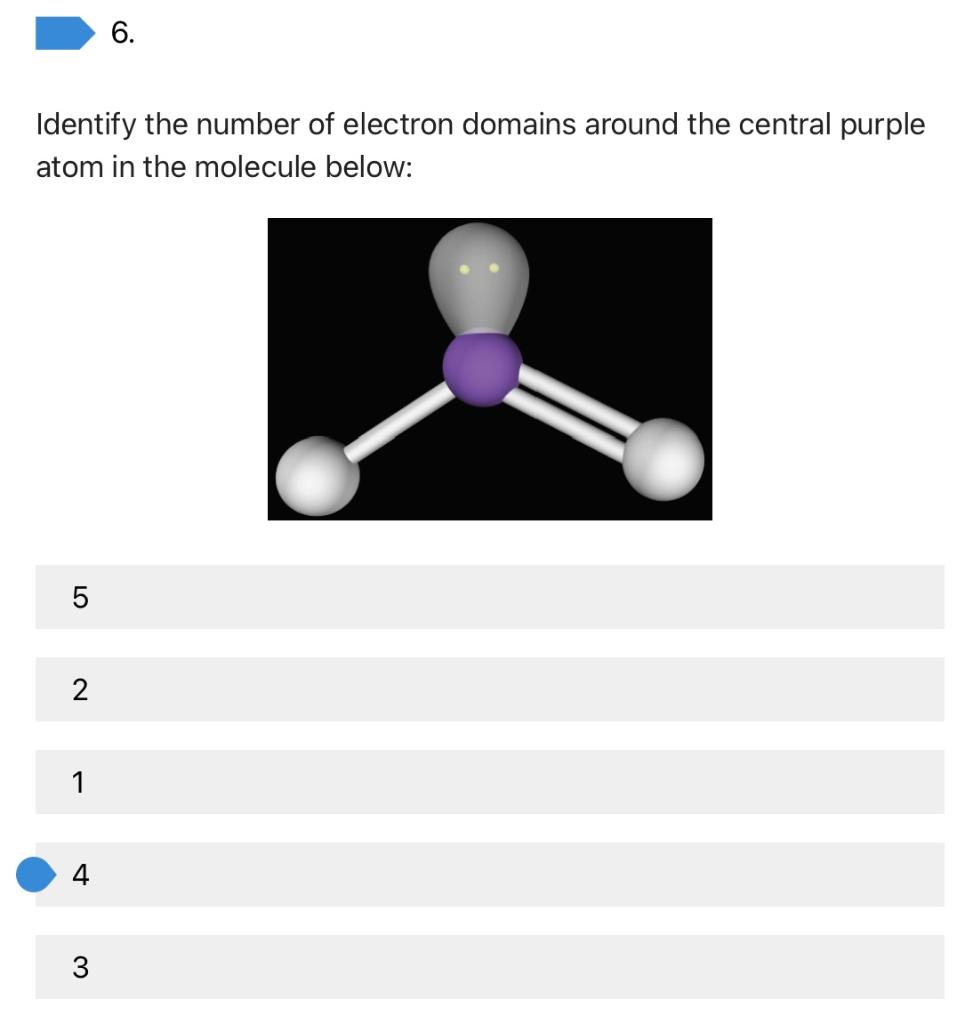
Solved 6. Identify the number of electron domains around the
An electron domain is the region of space around an atom where one or more electrons are found. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. Electron domains refer to.
Ch 9 Electron Domains & Molecular Geometries 1 YouTube
Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. An electron domain is the region of space around an atom where one or more electrons are found. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs,.
How to Determine the Number of Electron Domains and the Molecular
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. It is a fundamental concept in the study of atomic. An electron domain is the region of space around an atom where one or more electrons are found. Learn how to use the electron domain theory to predict the shapes and.
THE VSEPR MODEL MOLECULAR GEOMETRY AND BONDING THEORIES CHEMISTRY
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. A lone pair, single, double, or triple. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be.
Electron Domain Definition and VSEPR Theory
Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. An electron domain is the region of space around an atom where one or more electrons are found. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons..
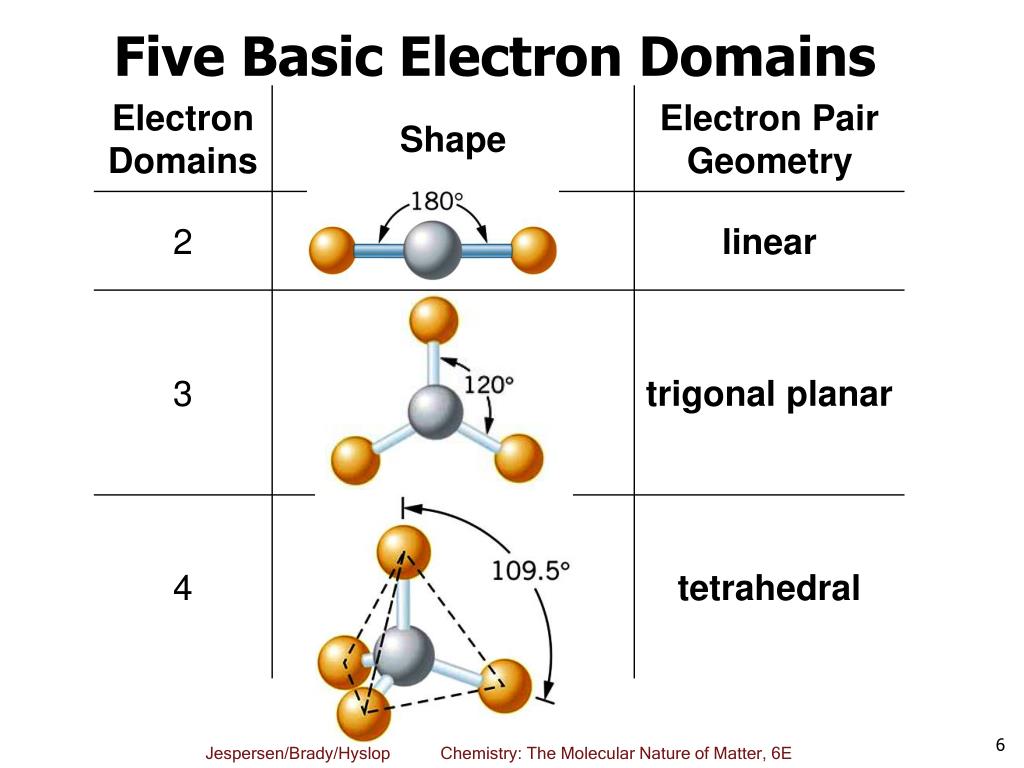
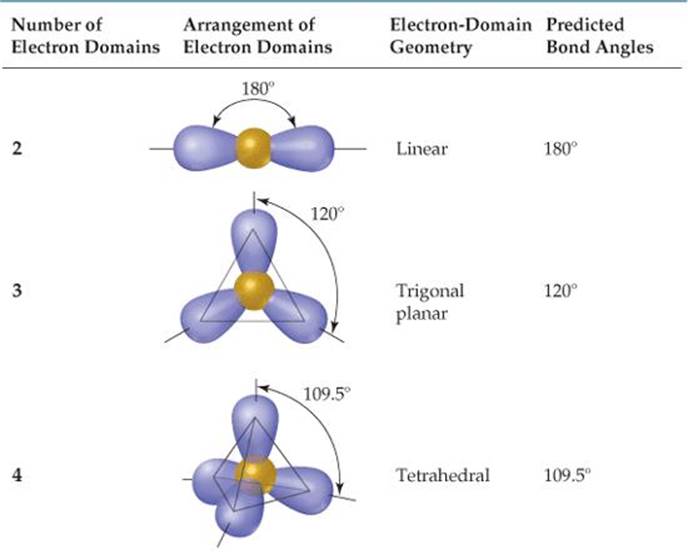
Electron Domain Geometry Chart
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. It is a fundamental concept in.
SOLVED(a) What is meant by the term electron domadit? (b) Explain in
Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. An electron domain is the region of space around an atom where one or more electrons are found. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how vsepr explains.
Electron Domain and Molecular Geometry Diagram Quizlet
Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. A lone pair, single, double, or triple. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. It is a fundamental concept in the study.
PPT Chapter 10 Theories of Bonding and Structure PowerPoint
Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. An electron domain is the region of space around an atom where one or more electrons are found. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or..
Electron Domains Are The Units Of.
A lone pair, single, double, or triple. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds.
Electron Domains Refer To Regions Around A Central Atom In A Molecule Where Electrons Are Likely To Be Found, Such As Bonding Pairs, Lone Pairs, Or.
An electron domain is the region of space around an atom where one or more electrons are found. It is a fundamental concept in the study of atomic.


/carbon-dioxide-molecule-545861181-5934474d3df78c08ab2ba23f.jpg)