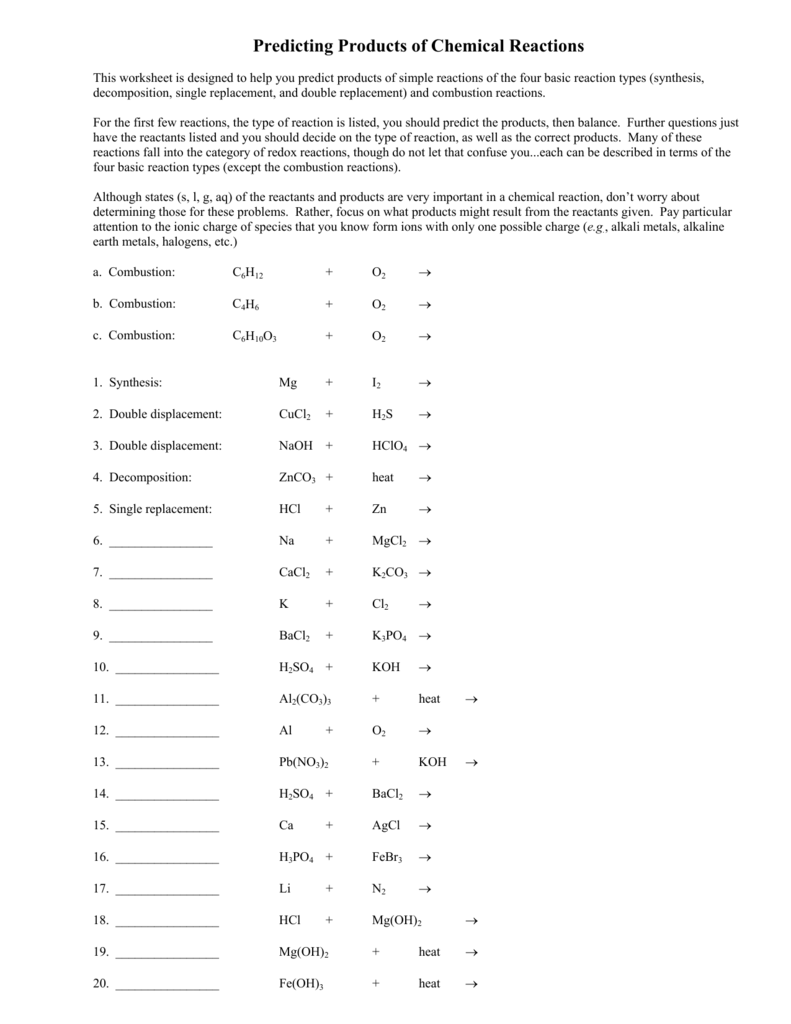
Predicting Products Of Chemical Reactions Worksheet - If a precipitate forms, indicate by circling the precipitate. Use your activity series, predicting products helper sheet and the solubility rules. Predict the products of the reactions below. Predict the products of the reactions below. Then, write the balanced equation and classify the reaction. Predict the products for, and then balance each of the following chemical reactions: Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Sii4 + mg → (single replacement) This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and.
Sii4 + mg → (single replacement) This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. If a precipitate forms, indicate by circling the precipitate. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Predict the products of the reactions below. Use your activity series, predicting products helper sheet and the solubility rules. Predict the products for, and then balance each of the following chemical reactions: Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Then, write the balanced equation and classify the reaction. Predict the products of the reactions below.
Then, write the balanced equation and classify the reaction. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Predict the products of the reactions below. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Use your activity series, predicting products helper sheet and the solubility rules. Sii4 + mg → (single replacement) If a precipitate forms, indicate by circling the precipitate. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Predict the products of the reactions below. Predict the products for, and then balance each of the following chemical reactions:
Types of Chemical Reactions Worksheets Free Printable
Then, write the balanced equation and classify the reaction. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Predict the products for, and then balance each of the following chemical reactions: Use your activity series, predicting products helper sheet and the solubility rules..
Predicting Products Of Chemical Reactions Worksheet Answers —
If a precipitate forms, indicate by circling the precipitate. Predict the products for, and then balance each of the following chemical reactions: Then, write the balanced equation and classify the reaction. Predict the products of the reactions below. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +.
Identifying Reaction Types Worksheet
Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Predict the products of the reactions below. Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. This worksheet is designed.
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
Use your activity series, predicting products helper sheet and the solubility rules. Sii4 + mg → (single replacement) Predict the products for, and then balance each of the following chemical reactions: Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement,.
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
Use your activity series, predicting products helper sheet and the solubility rules. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. If a precipitate forms, indicate by circling the precipitate. Predict the products for, and then balance each of the following chemical reactions: Predict the products of the reactions below.
Predicting Products Of Reactions Chem Worksheet 10 4 Answer Key — db
Predict the products of the reactions below. Predict the products for, and then balance each of the following chemical reactions: Sii4 + mg → (single replacement) Then, write the balanced equation and classify the reaction. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +.
Predicting The Products Of Chemical Reactions Worksheets
Then, write the balanced equation and classify the reaction. Predict the products of the reactions below. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. If a precipitate forms, indicate by circling the precipitate. Sii4 + mg → (single replacement)
Predicting Products of Chemical Reactions
If a precipitate forms, indicate by circling the precipitate. Predict the products of the reactions below. Then, write the balanced equation and classify the reaction. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride → zinc nitrate +.
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
If a precipitate forms, indicate by circling the precipitate. Use your activity series, predicting products helper sheet and the solubility rules. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Then, write the balanced equation and classify the reaction. Silver nitrate + zinc.
Chemical Reactions Worksheet Answers Predicting —
Use your activity series, predicting products helper sheet and the solubility rules. Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride.
Predict The Products Of The Reactions Below.
Predict the products for, and then balance each of the following chemical reactions: This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Sii4 + mg → (single replacement) If a precipitate forms, indicate by circling the precipitate.
Use Your Activity Series, Predicting Products Helper Sheet And The Solubility Rules.
Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Then, write the balanced equation and classify the reaction.