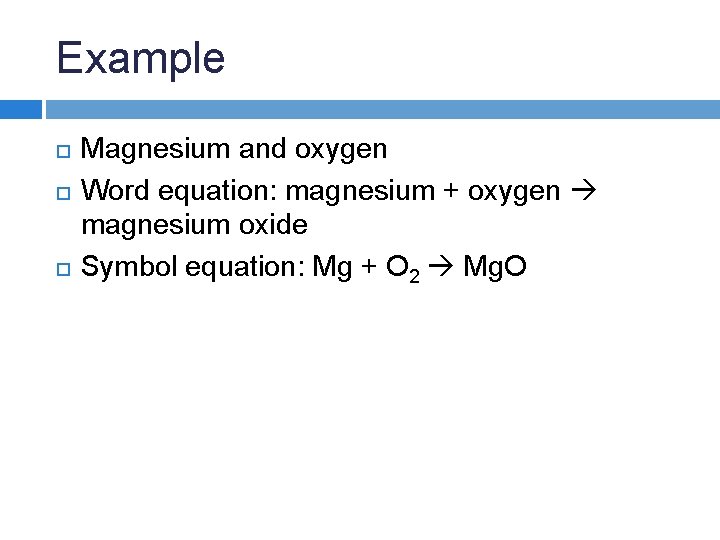
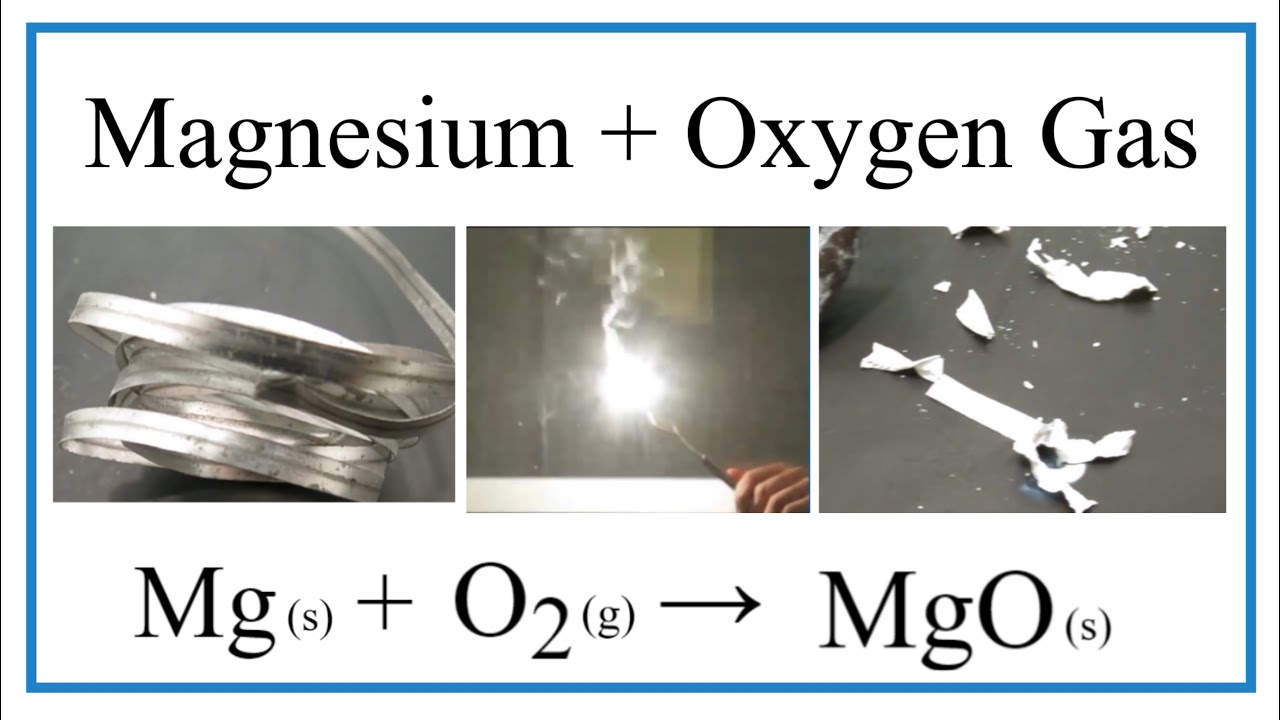
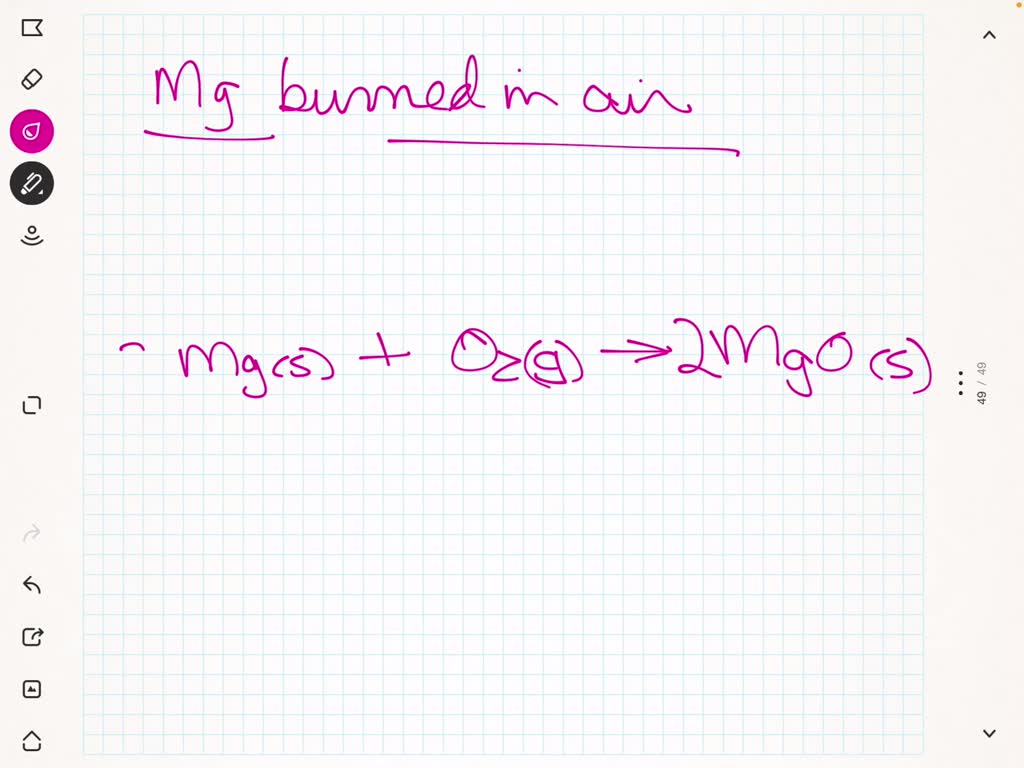Magnesium Oxygen Word Equation - Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. The word and symbol equations are: One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + oxygen = magnesium oxide. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium + dioxygen. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + dioxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. For example, magnesium and oxygen in the air react to produce magnesium oxide.
For example, magnesium and oxygen in the air react to produce magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + dioxygen = magnesium oxide. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. The word and symbol equations are: Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + oxygen = magnesium + dioxygen. Magnesium + oxygen = magnesium oxide. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and.
One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + dioxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium oxide. The word and symbol equations are: Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Magnesium + oxygen = magnesium + dioxygen. For example, magnesium and oxygen in the air react to produce magnesium oxide. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and.
CHEMICAL EQUATIONS Chemical equations You are expected to
Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium + dioxygen. The word and symbol equations are: Mg + o2 = mgo is a.
Simple Composition Reaction of magnesium and oxygen YouTube
Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium oxide. Magnesium + oxygen = magnesium + dioxygen. For example, magnesium and oxygen in the air react to produce magnesium oxide. Magnesium + dioxygen = magnesium oxide.
Write a word equation to show what happens when magnesium burns in
Magnesium + oxygen = magnesium oxide. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. For example, magnesium and oxygen in the air react to produce magnesium oxide. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. The word and symbol equations are:
Solved Balanced equation for magnesium burning in oxygen
Magnesium + oxygen = magnesium + dioxygen. Magnesium + oxygen = magnesium oxide. For example, magnesium and oxygen in the air react to produce magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are:
magnesium oxygen reaction
The word and symbol equations are: Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of.
What happens when Magnesium oxide is dissolved in water? Write a word
Magnesium + oxygen = magnesium oxide. Magnesium + dioxygen = magnesium oxide. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. The word and symbol equations are:
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen
Magnesium + dioxygen = magnesium oxide. Magnesium + oxygen = magnesium oxide. For example, magnesium and oxygen in the air react to produce magnesium oxide. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. The word and symbol equations are:
Reactions & Formulas
Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. This is because in a chemical reaction, the.
Fun Word Equation For Magnesium And Oxygen Mlt Dimensions Table
One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. For example, magnesium and oxygen in the air react to produce magnesium oxide. Since one molecule of o₂ contains two oxygen atoms, we need two magnesium atoms to react with one molecule of oxygen. Magnesium + oxygen = magnesium oxide. The word and.
SOLVED A strip of Magnesium ribbon should be held in the flame of a
For example, magnesium and oxygen in the air react to produce magnesium oxide. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + oxygen = magnesium oxide. This is because in a chemical reaction, the reactants (in this case, magnesium. Since one molecule of o₂ contains two oxygen atoms, we need.
Magnesium + Oxygen = Magnesium Oxide.
Mg + o2 = mgo is a synthesis reaction where two moles of magnesium [mg] and. This is because in a chemical reaction, the reactants (in this case, magnesium. The word and symbol equations are: Mg + o = mgo is a synthesis reaction where one mole of magnesium [mg] and one mole of.
Since One Molecule Of O₂ Contains Two Oxygen Atoms, We Need Two Magnesium Atoms To React With One Molecule Of Oxygen.
Magnesium + oxygen = magnesium + dioxygen. One mole of magnesium [mg] and two moles of oxygen [o] react to form one mole of. Magnesium + dioxygen = magnesium oxide. For example, magnesium and oxygen in the air react to produce magnesium oxide.