Li Ion Discharge - At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. The superior performance is achieved in part by. Monitoring these characteristics is vital for efficient. Lead acid discharges to 1.75v/cell;
Lead acid discharges to 1.75v/cell; Monitoring these characteristics is vital for efficient. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. The superior performance is achieved in part by. At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to.
Monitoring these characteristics is vital for efficient. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. The superior performance is achieved in part by. Lead acid discharges to 1.75v/cell; At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to.
a Schematic of Liion battery chargedischarge cycling. b Schematic of
A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. Lead acid discharges to 1.75v/cell; During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. Monitoring these characteristics is vital for efficient. At this level, roughly 95 percent of the energy is.
Schematic illustration of the charge/discharge process in a lithiumion
The superior performance is achieved in part by. Lead acid discharges to 1.75v/cell; A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. At this level, roughly 95 percent of the energy.
i Chargedischarge process of a lithiumion cell using graphite and
During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to..
Schematic diagram of the chargedischarge process of a Liion cell
Lead acid discharges to 1.75v/cell; Monitoring these characteristics is vital for efficient. At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge and.
The discharge process in a lithium ion battery cell and the
Lead acid discharges to 1.75v/cell; Monitoring these characteristics is vital for efficient. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. A standard operating temperature of 25±2°c during charge and.
Schematic illustration of a lithiumion battery during discharge/charge
A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. The superior performance is achieved in part by. Lead acid discharges to 1.75v/cell; At this level, roughly 95 percent of the energy.
Lithium Ion Battery Mechanism
During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet..
LiIon battery chargedischarge curve. Download Scientific Diagram
Lead acid discharges to 1.75v/cell; During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. Monitoring these characteristics is vital for efficient. The superior performance is achieved in part by.
How to read battery discharge curves Battery Power Tips
Lead acid discharges to 1.75v/cell; At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. The superior performance is achieved in part by. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. A standard operating temperature of 25±2°c during charge.
Schematic illustration of a lithiumion battery (LIB) under discharge
During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric. Lead acid discharges to 1.75v/cell; At this level, roughly 95 percent of the energy is spent, and the voltage would drop rapidly if the discharge were to. The superior performance is achieved in part by. A standard operating temperature of 25±2°c during charge.
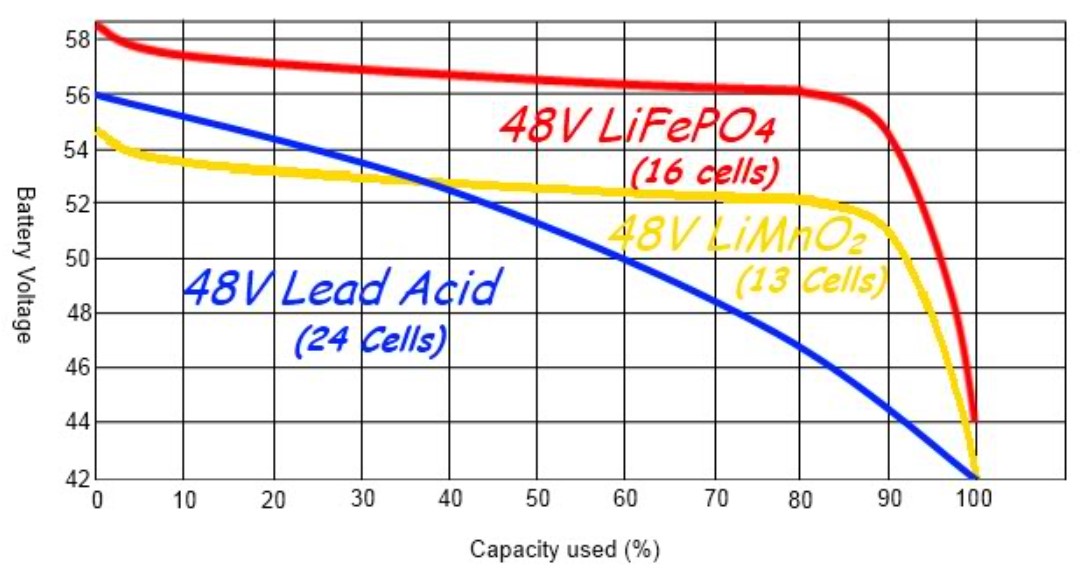
Lead Acid Discharges To 1.75V/Cell;
A standard operating temperature of 25±2°c during charge and discharge allows for the performance of the cell as per its datasheet. Monitoring these characteristics is vital for efficient. The superior performance is achieved in part by. During the discharging cycle, the lithium ions flow from the anode to the cathode, generating an electric.