In Every Chemical Reaction What Is Conserved - Let’s analyze a few rows of the above table, beginning with row a. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. This is conserved in every ordinary chemical reaction. Picture the reactants n 2 and h 2 as being the initial. The reactant that determines the amount of product that can be formed in a reaction. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants.
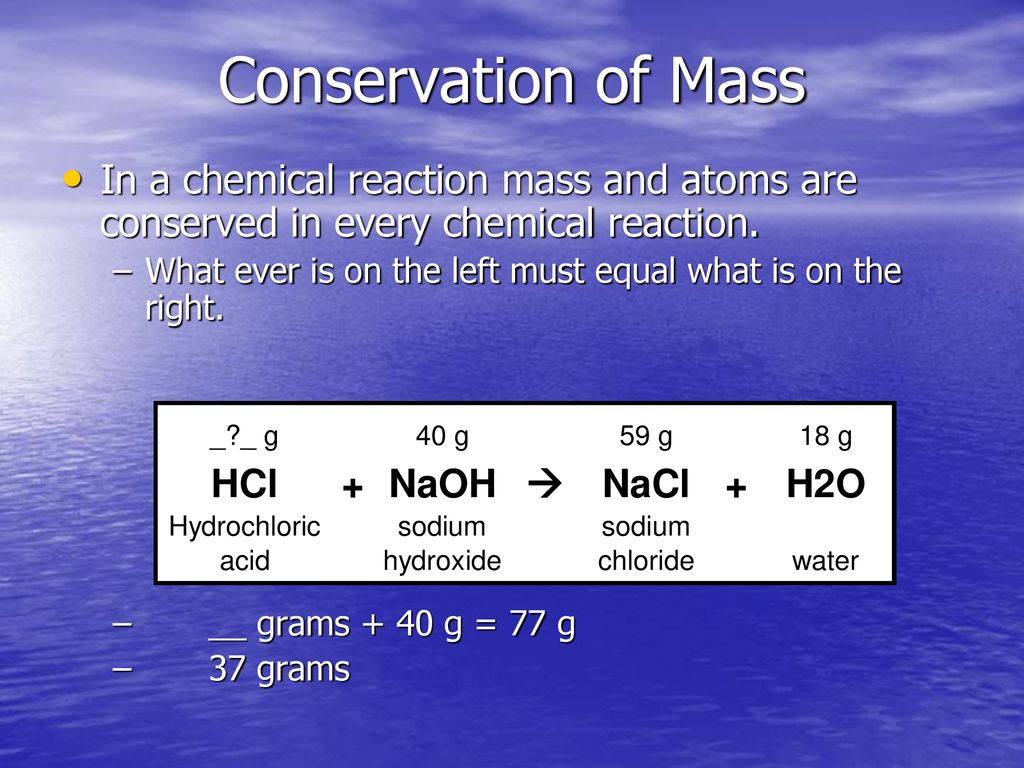
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. This is conserved in every ordinary chemical reaction. Let’s analyze a few rows of the above table, beginning with row a. The reactant that determines the amount of product that can be formed in a reaction. Picture the reactants n 2 and h 2 as being the initial. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants.
The reactant that determines the amount of product that can be formed in a reaction. Picture the reactants n 2 and h 2 as being the initial. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. This is conserved in every ordinary chemical reaction. Let’s analyze a few rows of the above table, beginning with row a.
What Is Conserved in Chemical Reactions? Sciencing
This is conserved in every ordinary chemical reaction. Picture the reactants n 2 and h 2 as being the initial. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Let’s analyze a few rows of the above table, beginning with row a. The law of conservation of matter says.
Stoichiometry Notes. In every chemical reaction, the mass and number of
Let’s analyze a few rows of the above table, beginning with row a. This is conserved in every ordinary chemical reaction. The reactant that determines the amount of product that can be formed in a reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the.
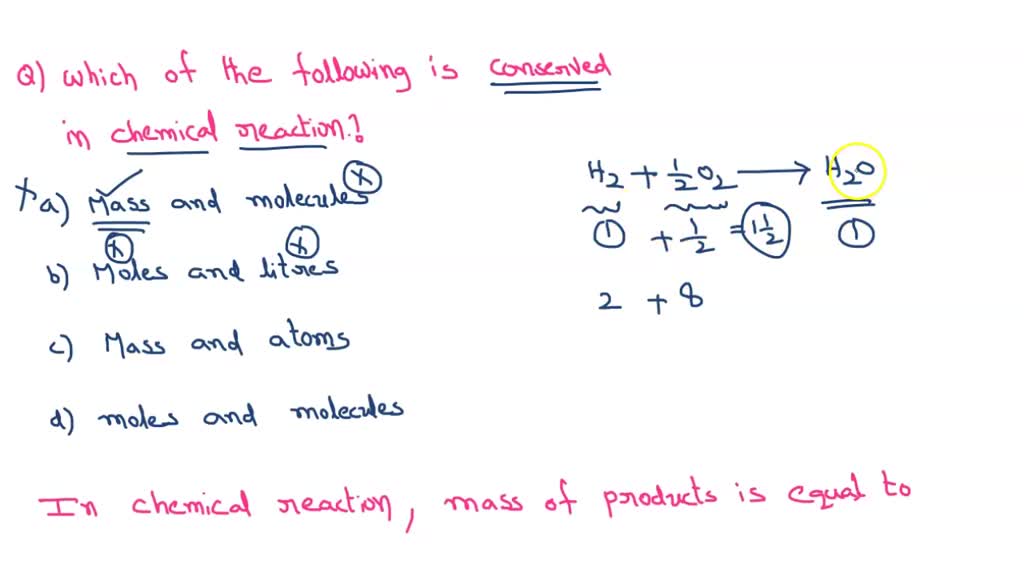
SOLVED Which of the following are CONSERVED in every chemical reaction
Picture the reactants n 2 and h 2 as being the initial. The reactant that determines the amount of product that can be formed in a reaction. This is conserved in every ordinary chemical reaction. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Let’s analyze a few rows.
Stoichiometry Chapter ppt download
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. Let’s analyze a few rows of the above table, beginning with row a. This is conserved in every ordinary chemical reaction. Picture the reactants n 2 and h 2 as being the initial. The reactant.
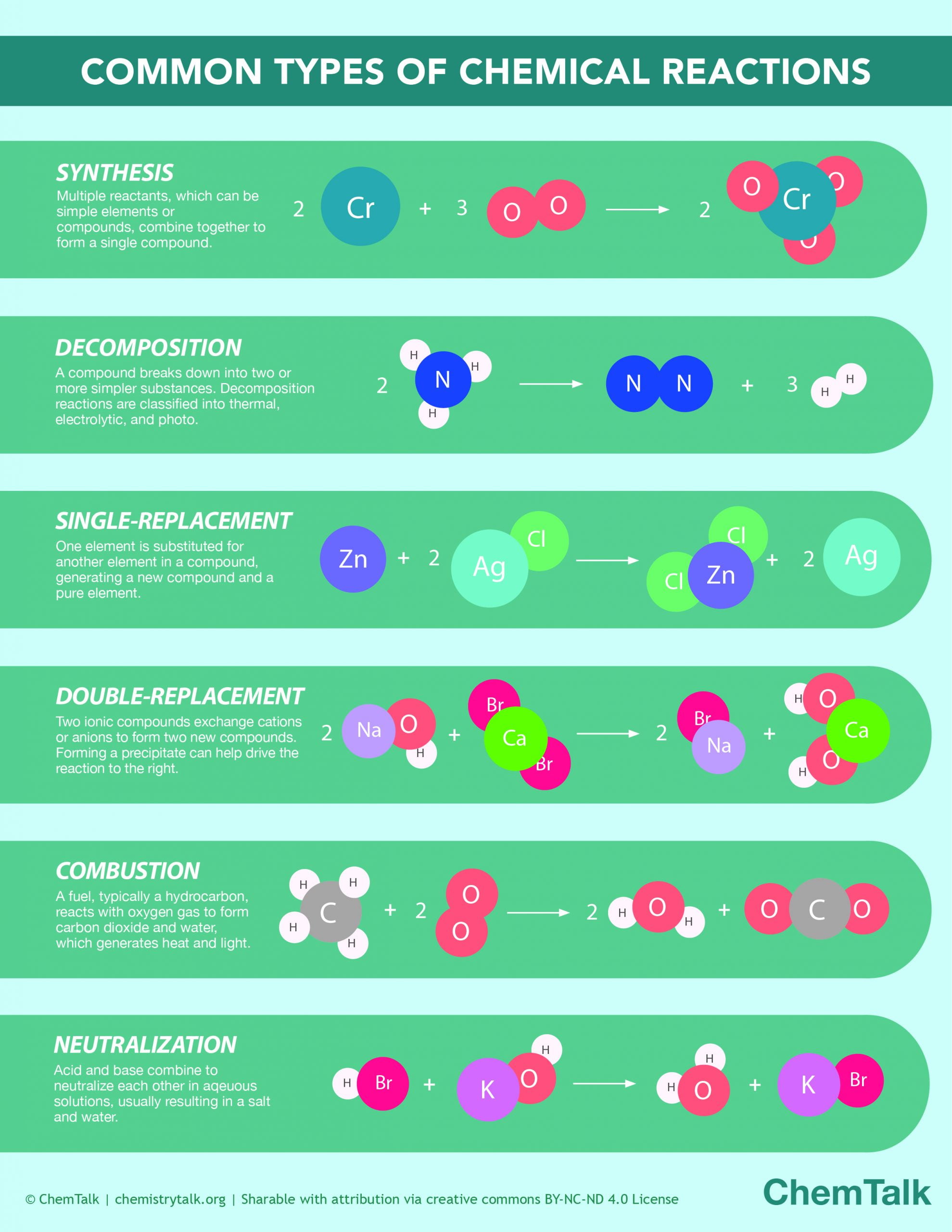
Chemical Reactions Infographic ChemTalk
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. This is conserved in every ordinary chemical reaction. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. The reactant that determines the amount of.
Stoichiometry moltomol ratios ppt download
The reactant that determines the amount of product that can be formed in a reaction. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants..
Stoichiometry ICS III Week ppt download
Picture the reactants n 2 and h 2 as being the initial. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. The reactant that.
Chemical Reaction Definition, Types and Examples Class 10 Science
The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. Let’s analyze a few rows of the above table, beginning with row a. Picture the reactants n 2 and h 2 as being the initial. In every chemical reaction, the same mass of matter must.
Review When converting FROM moles you MULTIPLY. ppt download
In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. Picture the reactants n 2 and h 2 as being the initial. This is conserved in every ordinary chemical reaction. Let’s analyze a few rows of the above table, beginning with row a. The law of conservation of matter says.
Drill What is a chemical reaction What is
Picture the reactants n 2 and h 2 as being the initial. The law of conservation of matter says that in chemical reactions, the total mass of the products must equal the total mass of the reactants. This is conserved in every ordinary chemical reaction. The reactant that determines the amount of product that can be formed in a reaction..
The Law Of Conservation Of Matter Says That In Chemical Reactions, The Total Mass Of The Products Must Equal The Total Mass Of The Reactants.
Let’s analyze a few rows of the above table, beginning with row a. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants. The reactant that determines the amount of product that can be formed in a reaction. This is conserved in every ordinary chemical reaction.