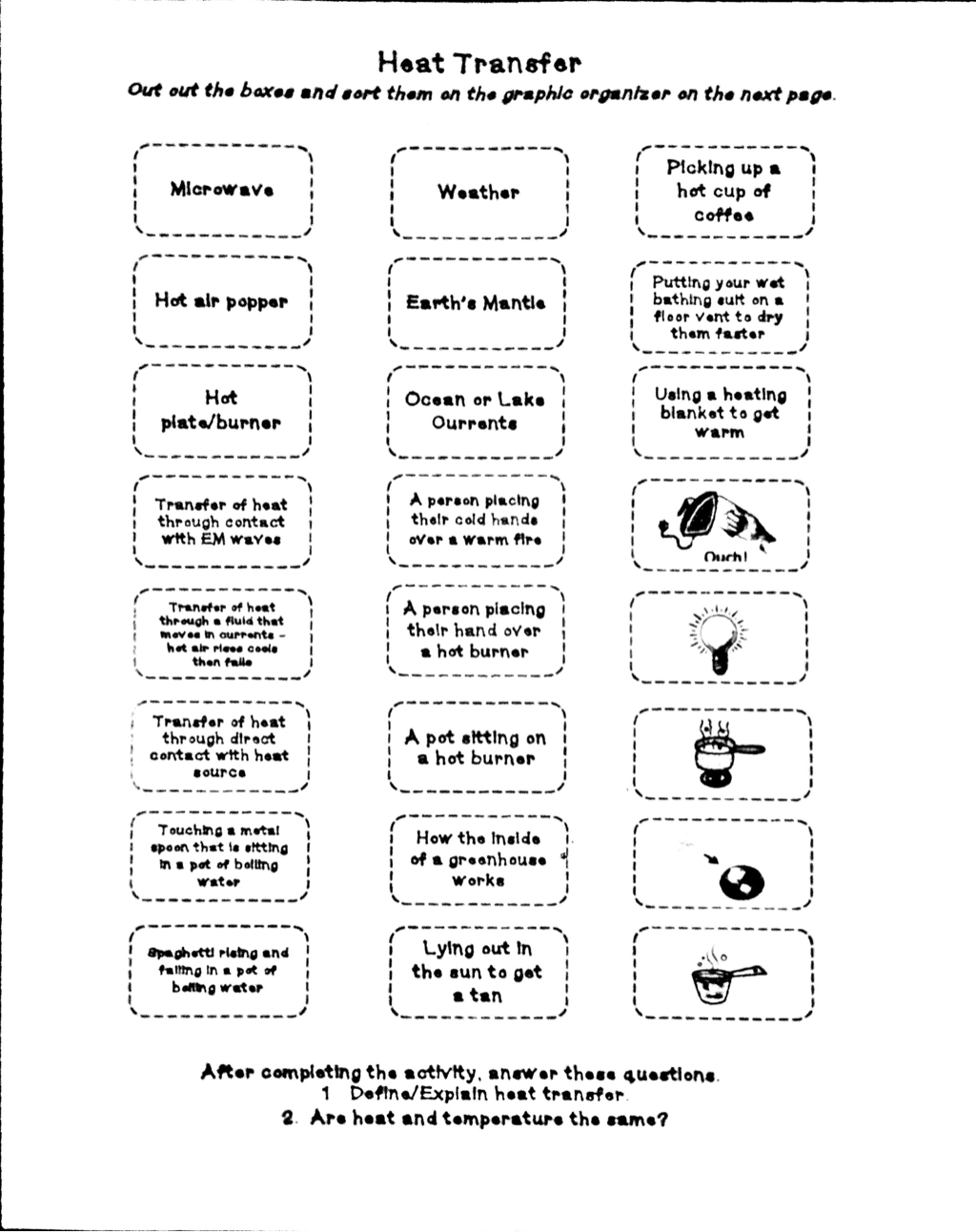
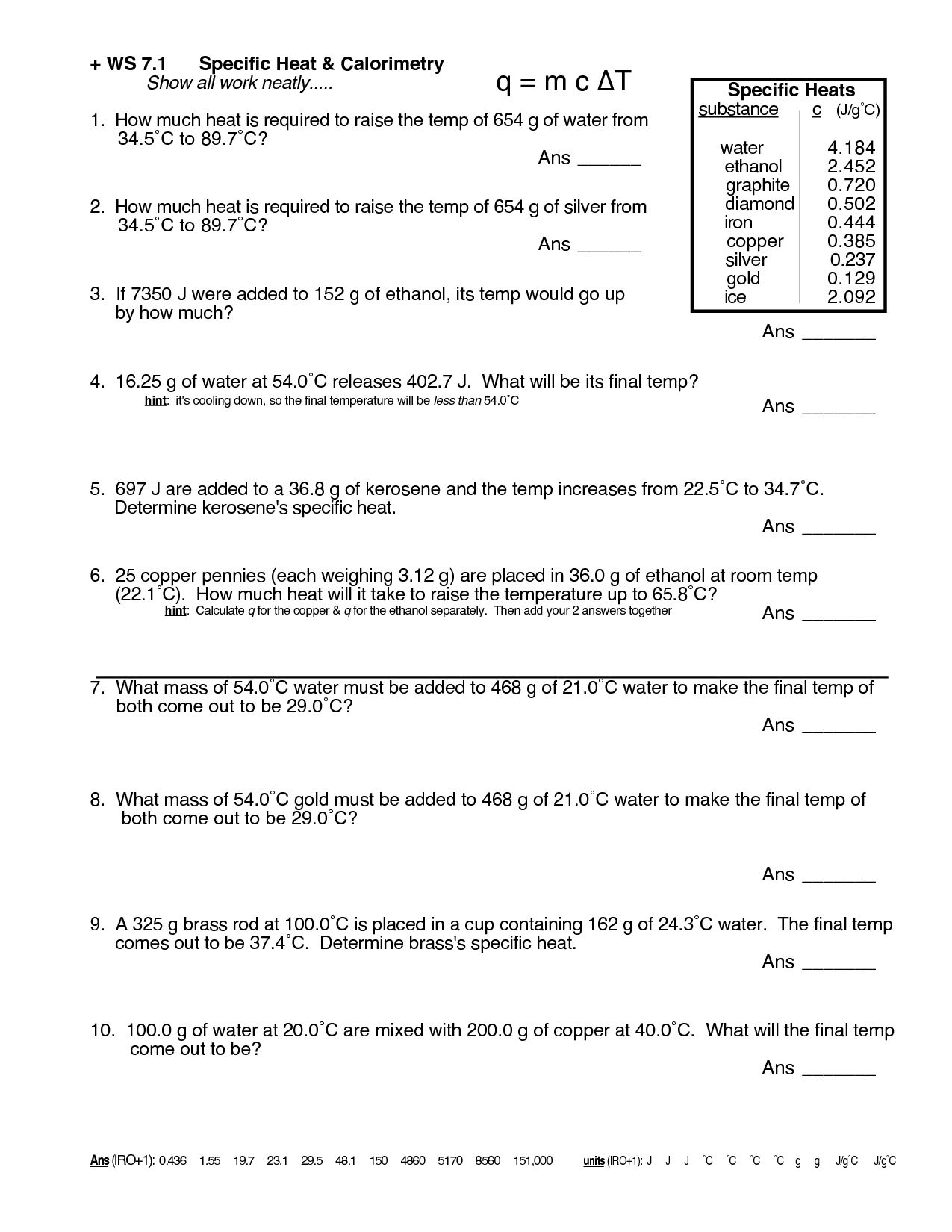
Heat Transfer Specific Heat Problems Worksheet - Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems.
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Show all work and units.
Heat Transfer Worksheet Answers Englishworksheet.my.id
Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems.
Heat Transfer Specific Heat Problems Worksheet —
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Specific Heat Worksheet Answers Master of Documents
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
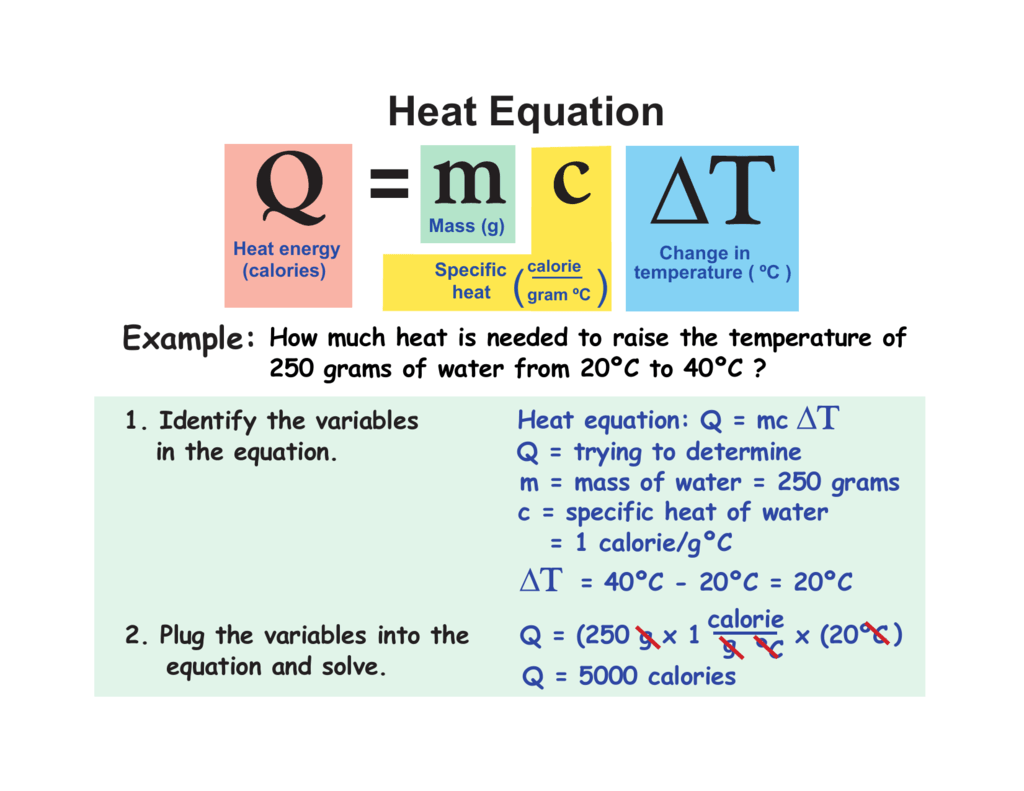
Heat Equation
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Heat Transfer Worksheet Answers E Street Light
Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Specific Heat Calculations Worksheet —
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Heat Transfer/ Specific Heat Problems Worksheet
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units.
Specific Heat Practice Problems Answers Page 1 of 3 Specific Heat
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Free specific heat and heat capacity worksheet, Download Free specific
Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Show All Work And Units.
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems.