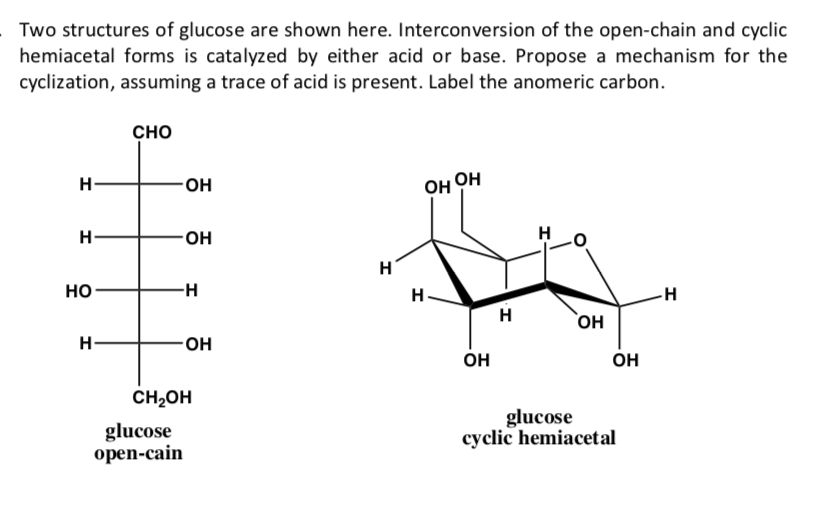
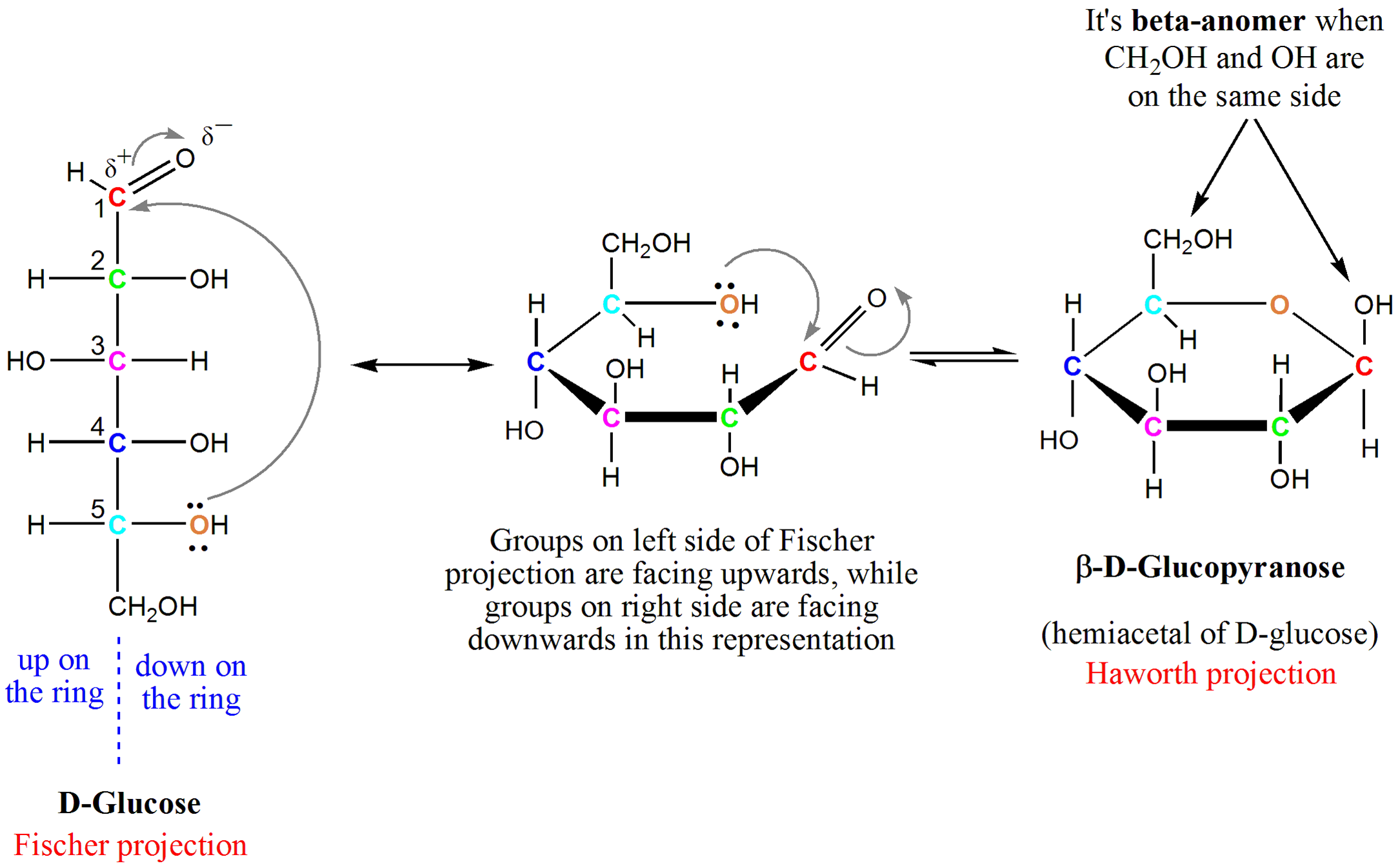
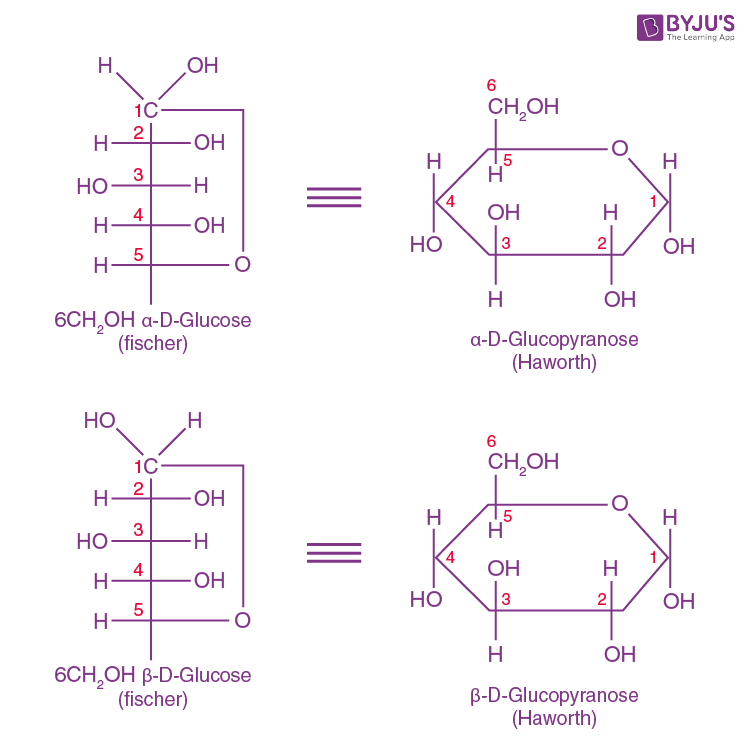
Glucose Forms A Cyclic Hemiacetal - Intramolecular hemiacetal formation is common in sugar chemistry. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more.
Intramolecular hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,.
A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more.
Solved Two structures of glucose are shown here.
For example, the common sugar glucose exists in the cylcic manner more. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry.
Epimers and Anomers Chemistry Steps
A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more.
11.3 Hemiacetals, hemiketals, and hydrates Chemistry LibreTexts
Intramolecular hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,.
SOLVED Draw the cyclic form of glucose in the following forms a
For example, the common sugar glucose exists in the cylcic manner more. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry.
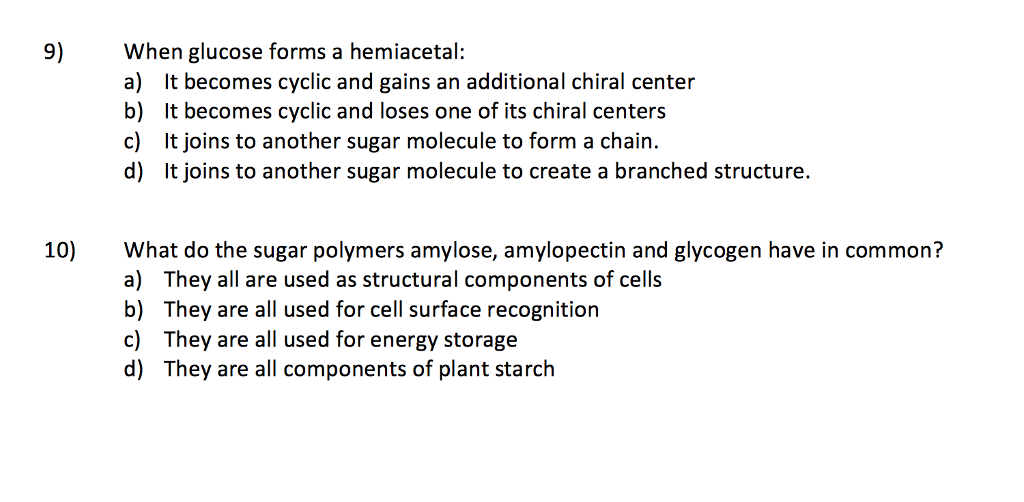
Solved 9 When glucose forms a hemiacetal a) It
For example, the common sugar glucose exists in the cylcic manner more. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry.
SOLVEDGlucose forms a cyclic hemiacetal in order to to prevent
Intramolecular hemiacetal formation is common in sugar chemistry. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more.
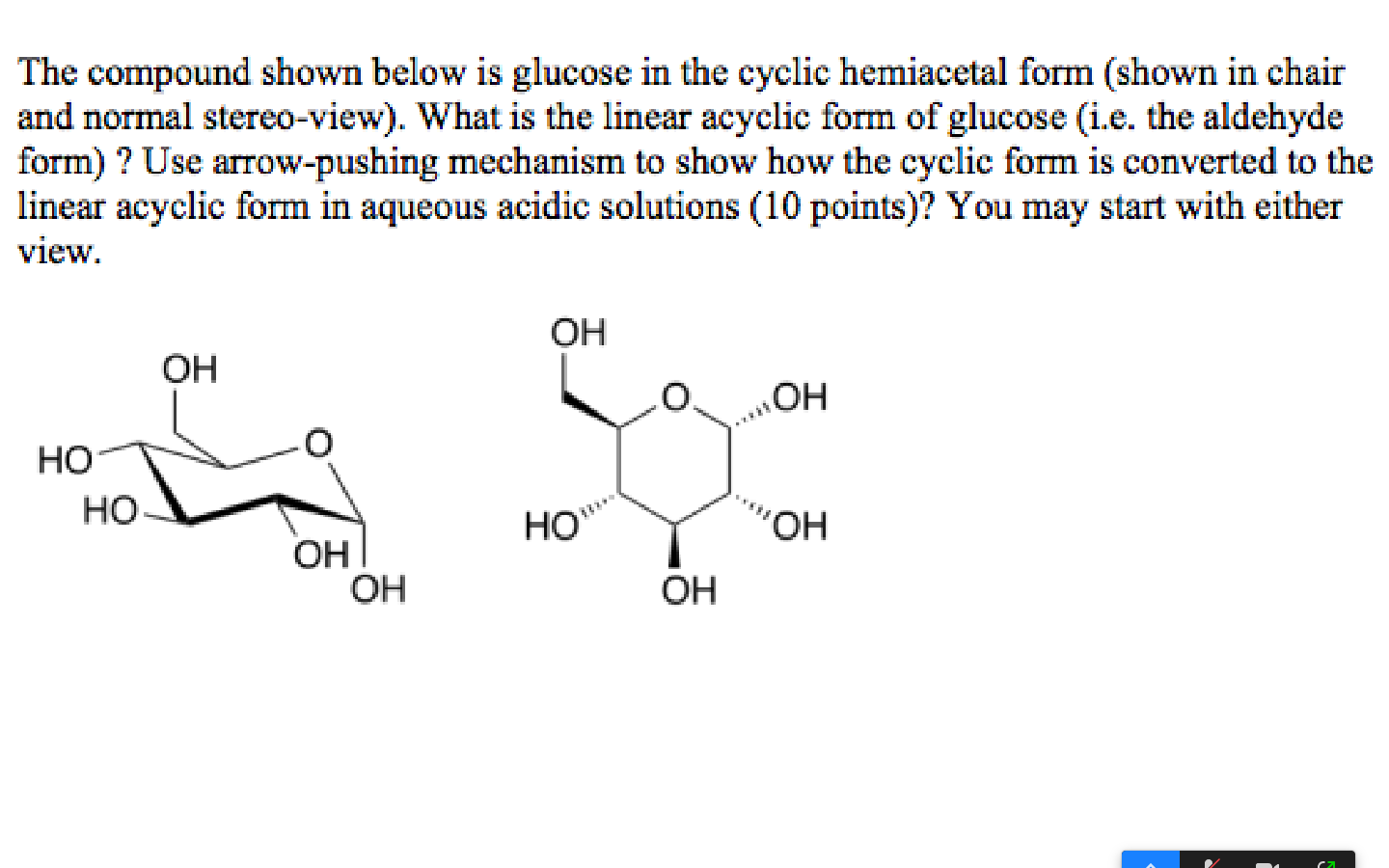
Solved The compound shown below is glucose in the cyclic
A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. Intramolecular hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more.
Glucose Ring Structure Formation
Intramolecular hemiacetal formation is common in sugar chemistry. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more.
What Is A Hemiacetal Sugar Peter Brown Bruidstaart
Intramolecular hemiacetal formation is common in sugar chemistry. A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more.
Chemical Structure Of Alpha D Glucose And Beta D Glucose,, 56 OFF
A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more. Intramolecular hemiacetal formation is common in sugar chemistry.
Intramolecular Hemiacetal Formation Is Common In Sugar Chemistry.
A simple solution to this dilemma is achieved by converting the open aldehyde structure for glucose into a cyclic hemiacetal,. For example, the common sugar glucose exists in the cylcic manner more.