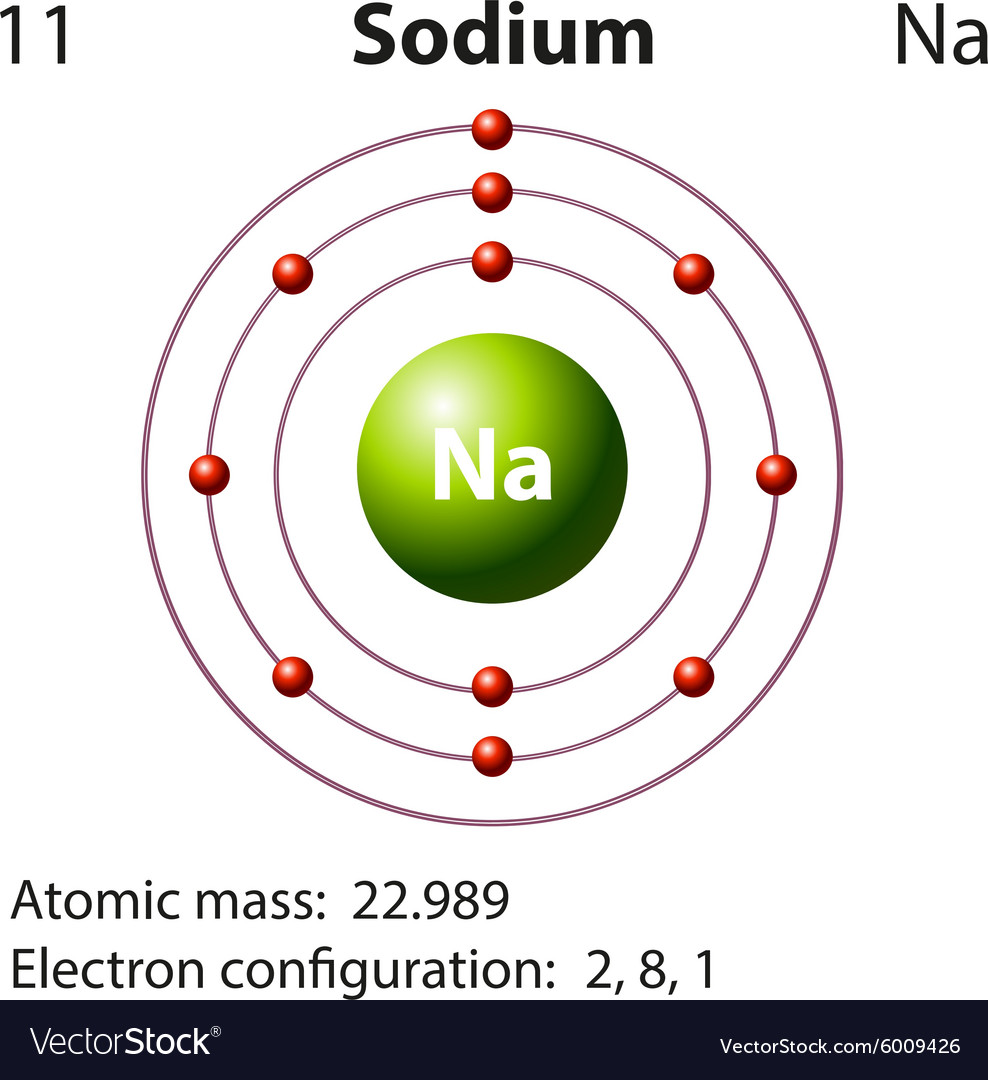
Full Electron Configuration For Sodium - What is the electron configuration for the element sodium? Electron configuration chart of all elements is mentioned in the table below. Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1. In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11 electrons). Sodium is one of the chemical elements that make up the periodic table, which is. The shorthand electron configuration (or noble gas. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell.
Sodium is one of the chemical elements that make up the periodic table, which is. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11 electrons). What is the electron configuration for the element sodium?
Sodium is one of the chemical elements that make up the periodic table, which is. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11 electrons). The electron configuration of sodium is 1s22s22p63s1. Sodium is an alkali metal with atomic number 11 and symbol na. What is the electron configuration for the element sodium? Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell.
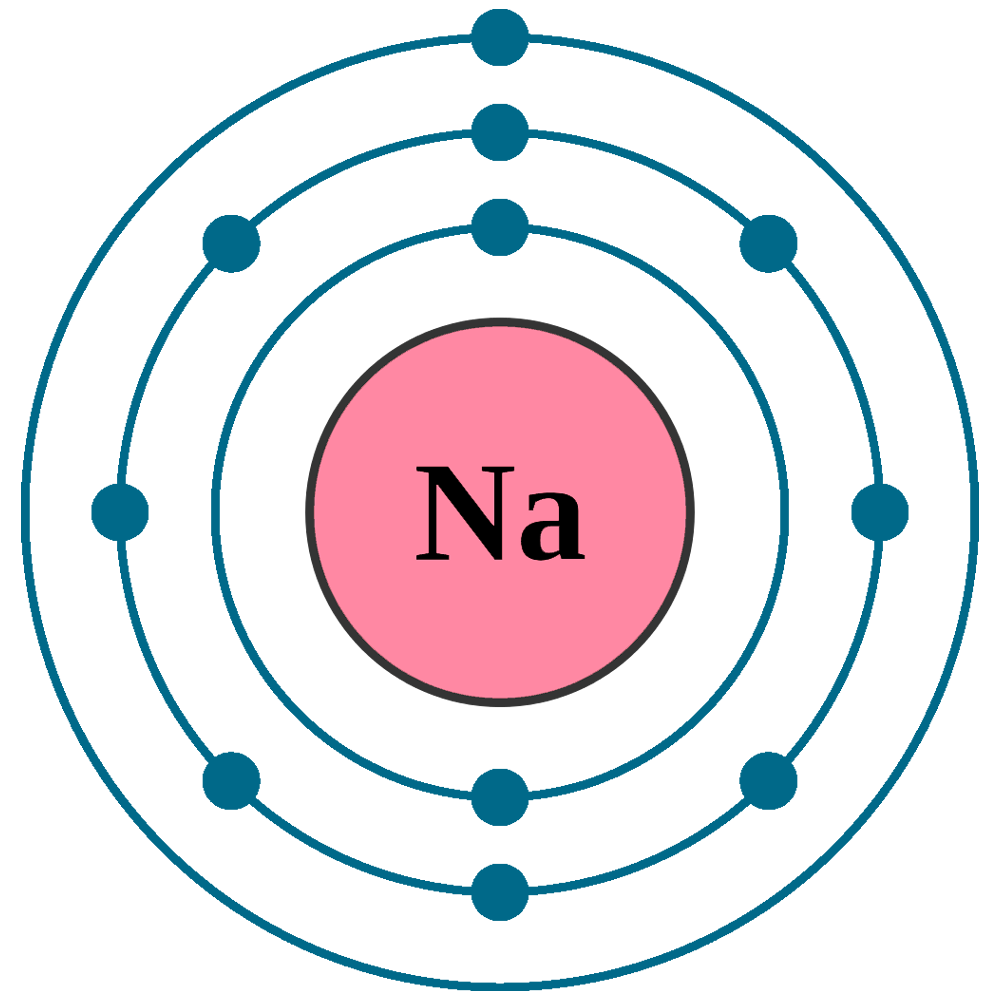
Diagram representation of the element sodium Vector Image
Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. Electron configuration chart of all elements is mentioned in the table below. What is the electron configuration for the element sodium? In order to write the na electron configuration we first need to know the number of electrons for the na atom (there.
Electron Configuration of Sodium, Na YouTube
Sodium is an alkali metal with atomic number 11 and symbol na. Sodium is one of the chemical elements that make up the periodic table, which is. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. What is the electron configuration for the element sodium? The shorthand electron configuration (or noble gas.
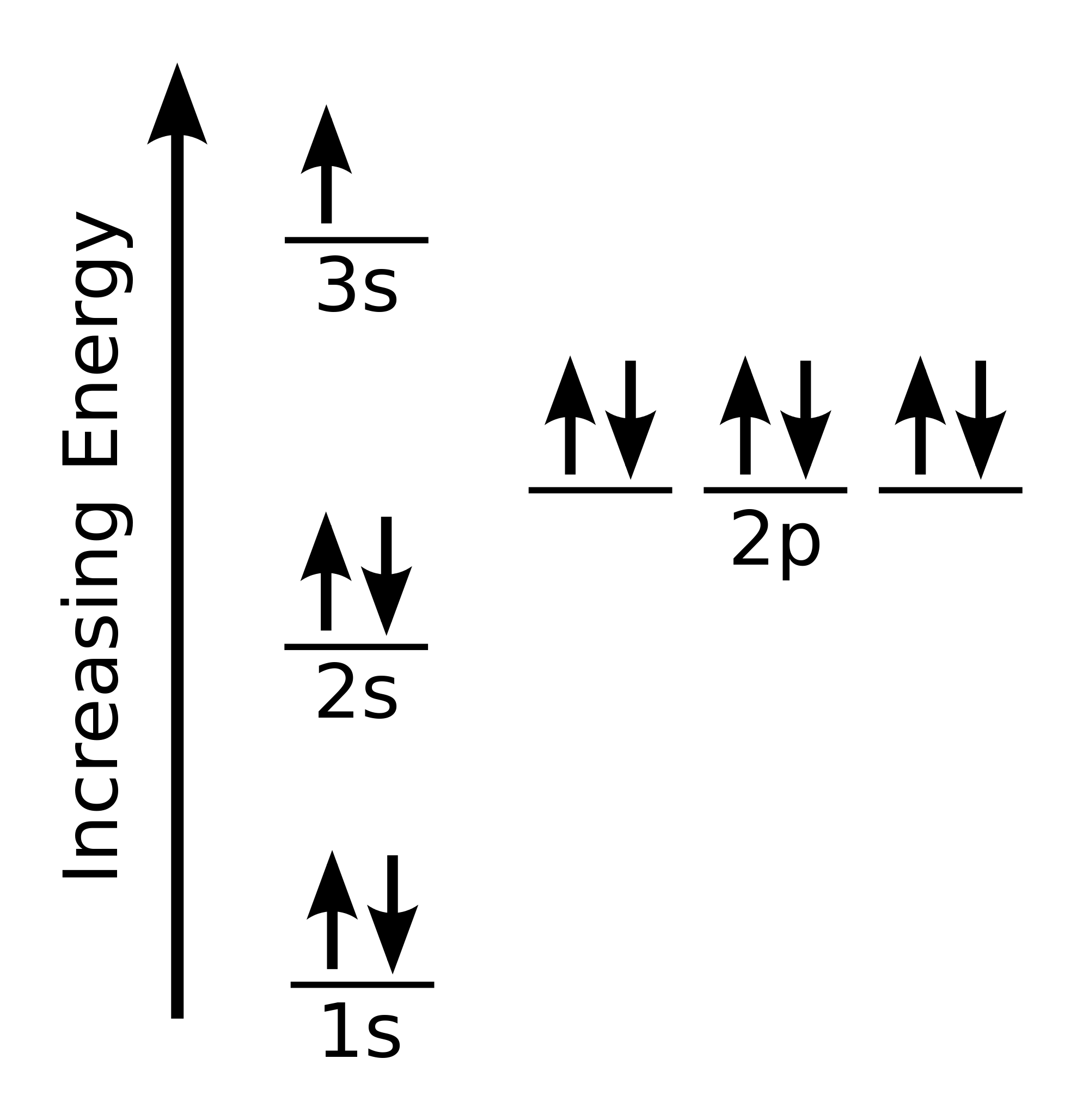
This is the electron configuration for sodium. Like ALL of the other
Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11.
How Many Valence Electrons Does Sodium(Na) Have?
In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11 electrons). What is the electron configuration for the element sodium? Sodium is an alkali metal with atomic number 11 and symbol na. The shorthand electron configuration (or noble gas. Its electron configuration is [ne] 3s 1,.
Create The Orbital Diagram For Sodium
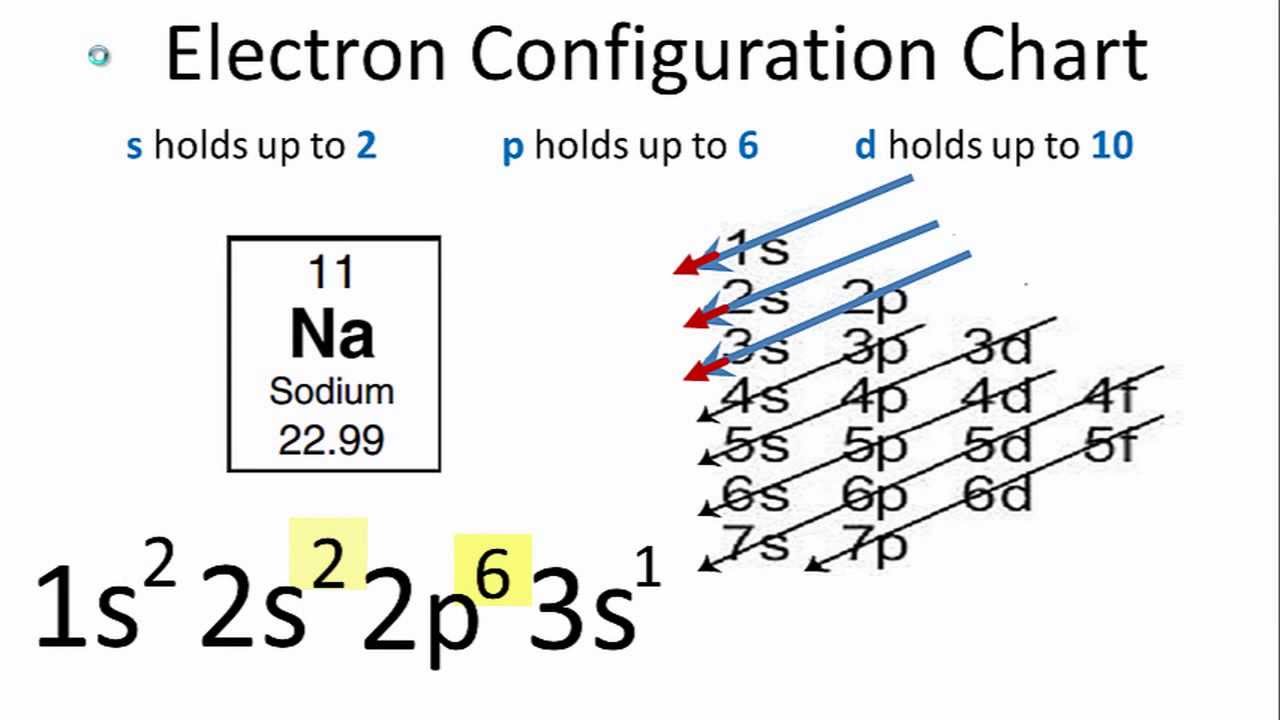
The electron configuration of sodium is 1s22s22p63s1. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. The shorthand electron configuration (or noble gas. Sodium is one of the chemical elements that make up the periodic table, which is. Sodium is an alkali metal with atomic number 11 and symbol na.
Question 2d40e Socratic
The shorthand electron configuration (or noble gas. The electron configuration of sodium is 1s22s22p63s1. Sodium is one of the chemical elements that make up the periodic table, which is. Sodium is an alkali metal with atomic number 11 and symbol na. What is the electron configuration for the element sodium?
sodium electron configuration Newton Desk
The electron configuration of sodium is 1s22s22p63s1. What is the electron configuration for the element sodium? Electron configuration chart of all elements is mentioned in the table below. Sodium is an alkali metal with atomic number 11 and symbol na. In order to write the na electron configuration we first need to know the number of electrons for the na.
Introduction to Atoms
Sodium is one of the chemical elements that make up the periodic table, which is. Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell. Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1. In order to write the na electron configuration we.
Sodium Electron Configuration YouTube
The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. Sodium is one of the chemical elements that make up the periodic table, which is. Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1.
How Many Valence Electrons Does Sodium(Na) Have?
The electron configuration of sodium is 1s22s22p63s1. The shorthand electron configuration (or noble gas. Sodium is an alkali metal with atomic number 11 and symbol na. What is the electron configuration for the element sodium? Electron configuration chart of all elements is mentioned in the table below.
Sodium Is One Of The Chemical Elements That Make Up The Periodic Table, Which Is.
Sodium is an alkali metal with atomic number 11 and symbol na. The electron configuration of sodium is 1s22s22p63s1. In order to write the na electron configuration we first need to know the number of electrons for the na atom (there are 11 electrons). The shorthand electron configuration (or noble gas.
Electron Configuration Chart Of All Elements Is Mentioned In The Table Below.
What is the electron configuration for the element sodium? Its electron configuration is [ne] 3s 1, which means it has one electron in its outer shell.