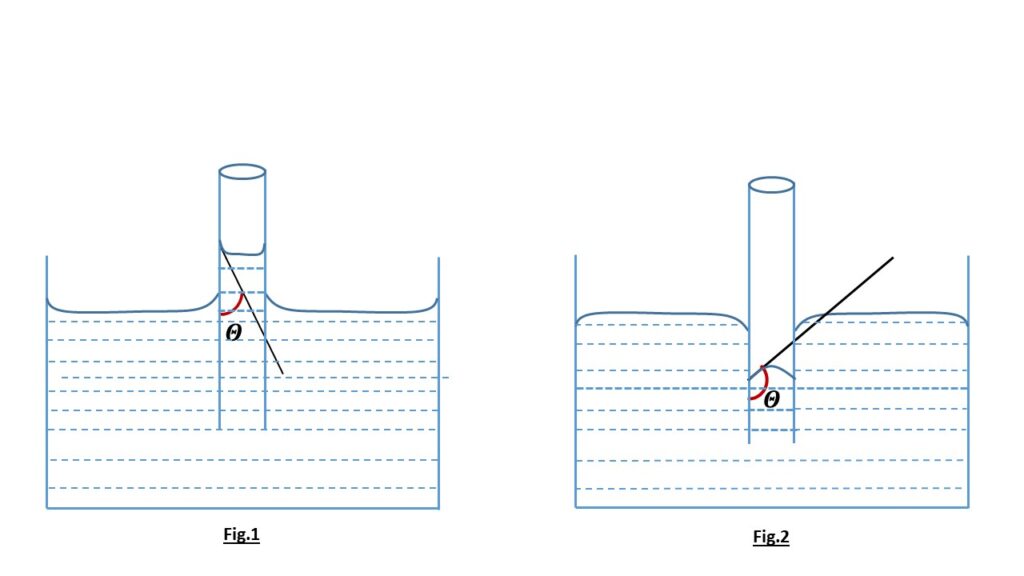
Explain Why Liquids In A Test Tube Form A Meniscus - (a) relatively high density, (b) ability to diffuse, and (c) ability to. It happens because the liquid sticks to the side of the container more than to itself. Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. A meniscus is a curved surface of a liquid in a container.
(a) relatively high density, (b) ability to diffuse, and (c) ability to. Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. It happens because the liquid sticks to the side of the container more than to itself. A meniscus is a curved surface of a liquid in a container.
Surface tension and adhesive forces cause the formation of a meniscus. A meniscus is a curved surface of a liquid in a container. (a) relatively high density, (b) ability to diffuse, and (c) ability to. It happens because the liquid sticks to the side of the container more than to itself. Why do liquids in a test tube form a meniscus?
SOLVED What is a meniscus? Why do some liquids develop a meniscus? How
(a) relatively high density, (b) ability to diffuse, and (c) ability to. Surface tension and adhesive forces cause the formation of a meniscus. It happens because the liquid sticks to the side of the container more than to itself. Why do liquids in a test tube form a meniscus? A meniscus is a curved surface of a liquid in a.
The liquid meniscus in a capillary tube will be convex, if the angle of
It happens because the liquid sticks to the side of the container more than to itself. Why do liquids in a test tube form a meniscus? (a) relatively high density, (b) ability to diffuse, and (c) ability to. A meniscus is a curved surface of a liquid in a container. Surface tension and adhesive forces cause the formation of a.
Understanding the Importance of the Meniscus A Crucial Aspect of
It happens because the liquid sticks to the side of the container more than to itself. Surface tension and adhesive forces cause the formation of a meniscus. Why do liquids in a test tube form a meniscus? (a) relatively high density, (b) ability to diffuse, and (c) ability to. A meniscus is a curved surface of a liquid in a.
For which of the following liquids, the liquid meniscus in the
It happens because the liquid sticks to the side of the container more than to itself. (a) relatively high density, (b) ability to diffuse, and (c) ability to. Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. A meniscus is a curved surface of a liquid in a.
Meniscus Science
Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. (a) relatively high density, (b) ability to diffuse, and (c) ability to. It happens because the liquid sticks to the side of the container more than to itself. A meniscus is a curved surface of a liquid in a.
Meniscus test tube hires stock photography and images Alamy
(a) relatively high density, (b) ability to diffuse, and (c) ability to. A meniscus is a curved surface of a liquid in a container. Why do liquids in a test tube form a meniscus? It happens because the liquid sticks to the side of the container more than to itself. Surface tension and adhesive forces cause the formation of a.
What Is The Shape Of A Liquid Meniscus In A Capillary Tube? Physics
Surface tension and adhesive forces cause the formation of a meniscus. (a) relatively high density, (b) ability to diffuse, and (c) ability to. A meniscus is a curved surface of a liquid in a container. Why do liquids in a test tube form a meniscus? It happens because the liquid sticks to the side of the container more than to.
SOLVED What is meniscus? Why do some liquids develop meniscus? How do
Why do liquids in a test tube form a meniscus? (a) relatively high density, (b) ability to diffuse, and (c) ability to. A meniscus is a curved surface of a liquid in a container. It happens because the liquid sticks to the side of the container more than to itself. Surface tension and adhesive forces cause the formation of a.
SOLVED 2. Write the formula for the energy (KE) of a moving
A meniscus is a curved surface of a liquid in a container. (a) relatively high density, (b) ability to diffuse, and (c) ability to. It happens because the liquid sticks to the side of the container more than to itself. Surface tension and adhesive forces cause the formation of a meniscus. Why do liquids in a test tube form a.
Adhesion Of Water Meniscus
A meniscus is a curved surface of a liquid in a container. It happens because the liquid sticks to the side of the container more than to itself. Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. (a) relatively high density, (b) ability to diffuse, and (c) ability.
A Meniscus Is A Curved Surface Of A Liquid In A Container.
(a) relatively high density, (b) ability to diffuse, and (c) ability to. Why do liquids in a test tube form a meniscus? Surface tension and adhesive forces cause the formation of a meniscus. It happens because the liquid sticks to the side of the container more than to itself.



:max_bytes(150000):strip_icc()/GettyImages-692027135-fdcc07eeb4604401beb7e020f0e5b310.jpg)