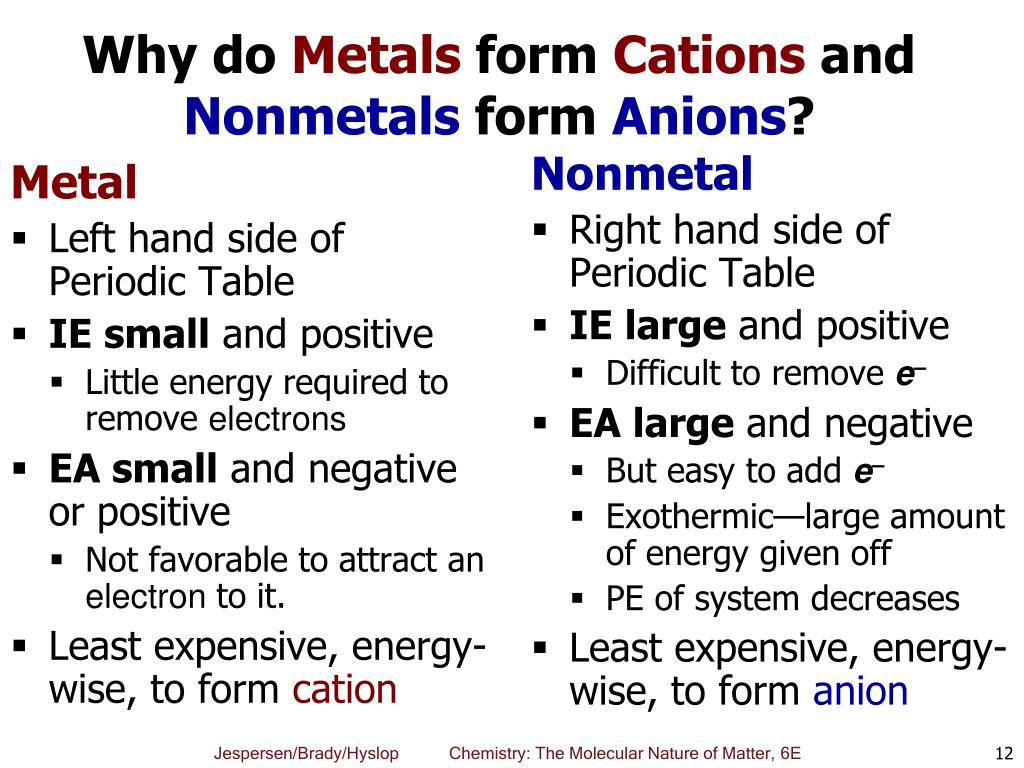
Do Metals Form Cations - Ionization energy is the energy required to remove. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they have low ionization energy and low electronegativity. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Virtually all of the transition metals form.
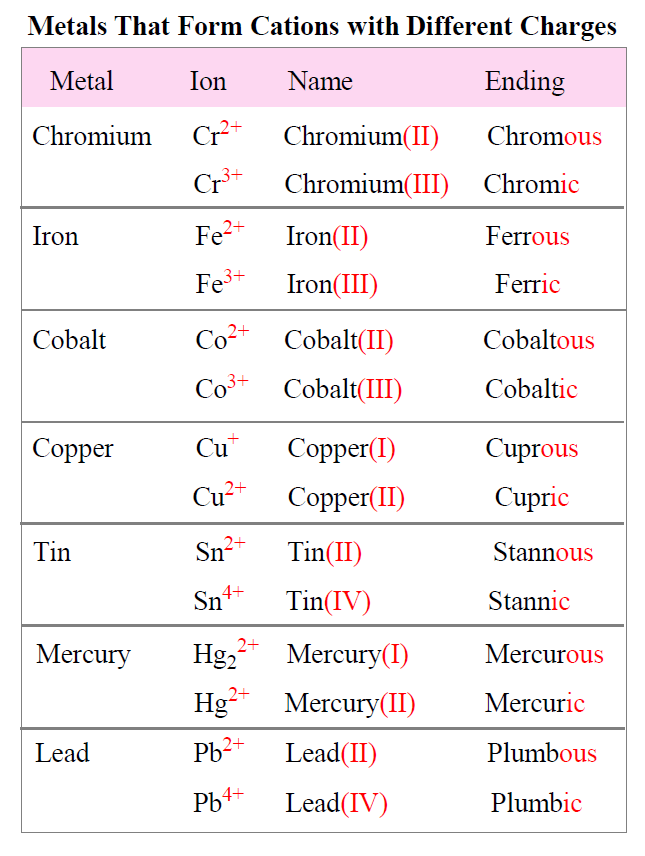
Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Ionization energy is the energy required to remove. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity.
Virtually all of the transition metals form. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Ionization energy is the energy required to remove. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Ionization energy is the energy required to remove..
Ionic Compounds Stone Cold Chemistry Talk
Ionization energy is the energy required to remove. Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge..
Do Metals Form Anions Or Cations
Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing.
PPT Notes for Oct 23 and Oct 24 PowerPoint Presentation, free
With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they have low ionization energy and low electronegativity. Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge..
Diferencia entre aniónes y catiónes (con nomenclatura y ejemplos
Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they.
H2 cation and anion Kangen Water Singapore HydrogenRich Water
Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Ionization.
Do Metals Form Anions Or Cations
Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Ionization energy is the energy required to remove. Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations because they.
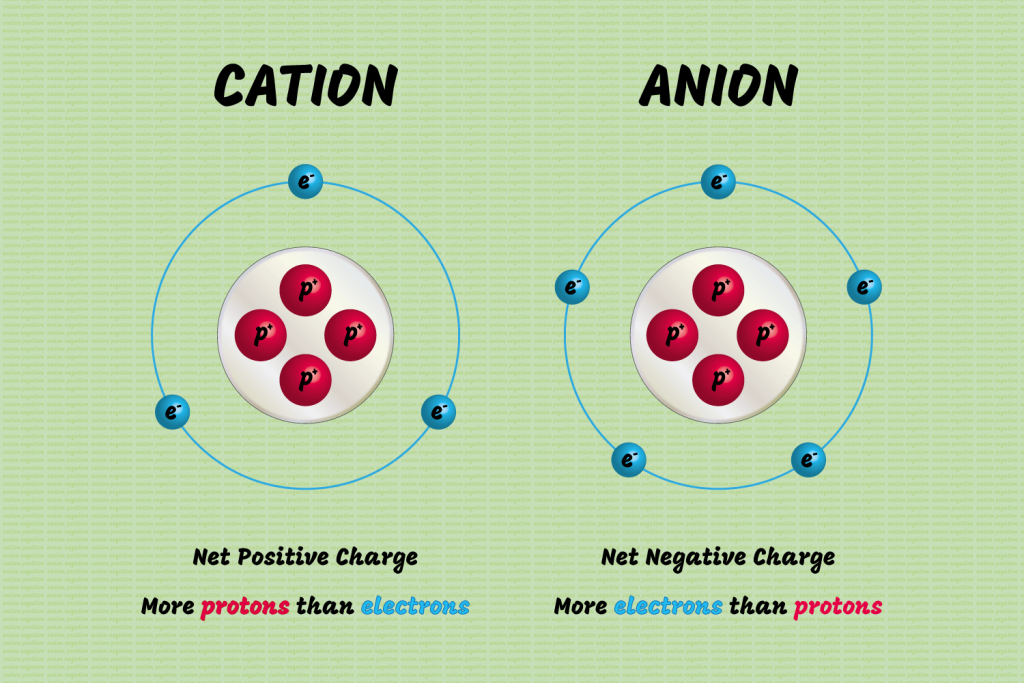
Cations vs Anions Difference Between Cations and Anions with Examples
Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they have low ionization energy and low electronegativity. Virtually all of the transition metals form. Ionization energy is the energy required to remove. With the exception of hydrogen, all elements that form positive ions by losing.
Naming Monatomic and Polyatomic Ions Chemistry Steps
Ionization energy is the energy required to remove. Virtually all of the transition metals form. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Metals tend to form cations because they.
Pin on What is an ion?
Ionization energy is the energy required to remove. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Most transition metals form multiple cations, that is, they have more.
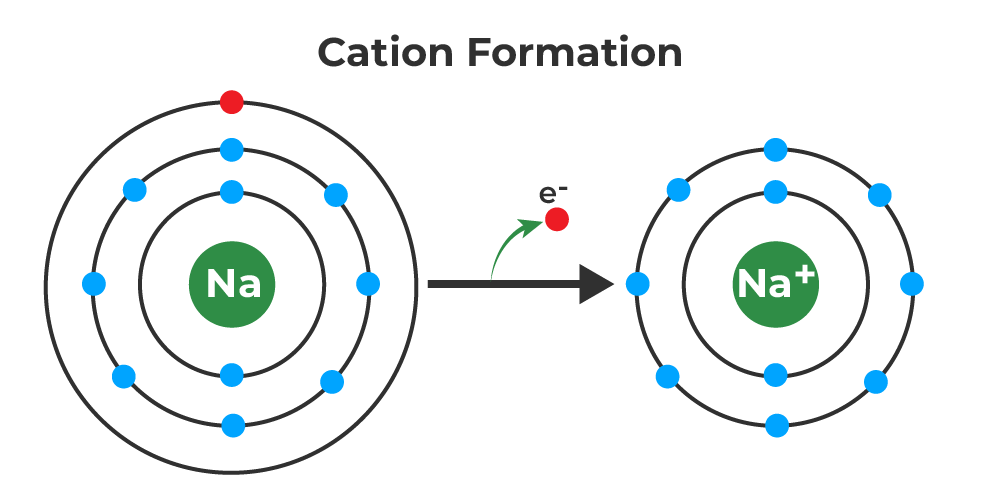
With The Exception Of Hydrogen, All Elements That Form Positive Ions By Losing Electrons During Chemical Reactions Are Called Metals.
Ionization energy is the energy required to remove. Most transition metals form multiple cations, that is, they have more than one possible amount of positive charge. Virtually all of the transition metals form. Metals tend to form cations because they have low ionization energy and low electronegativity.