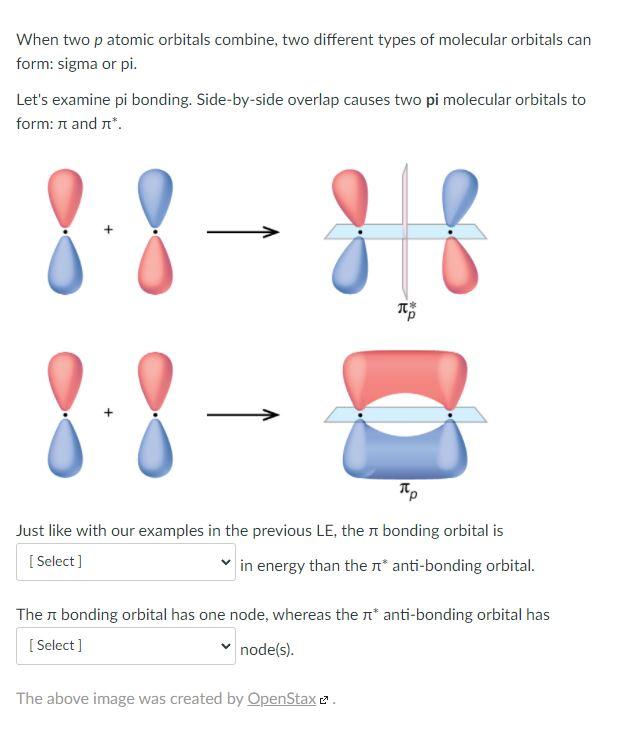
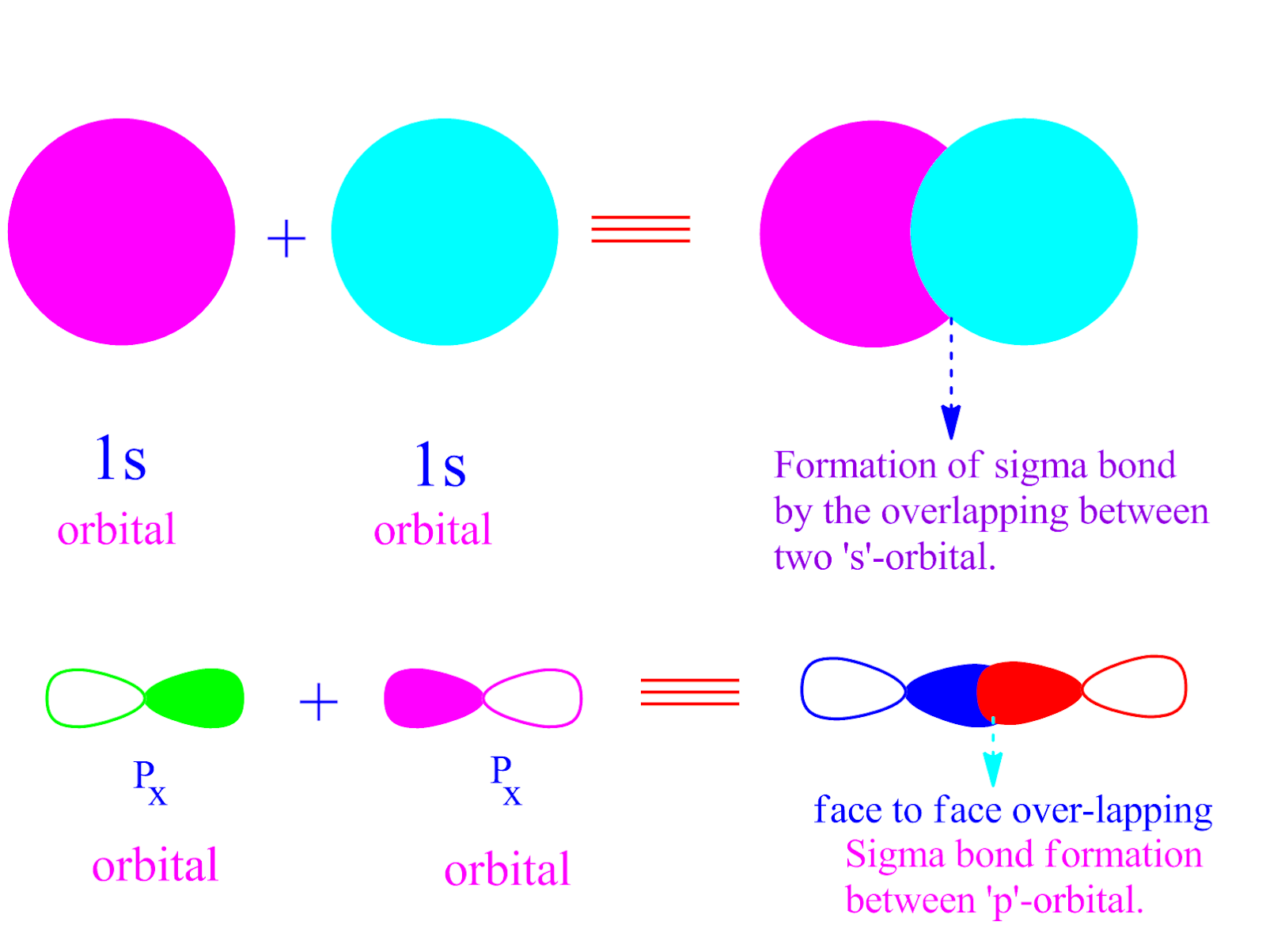
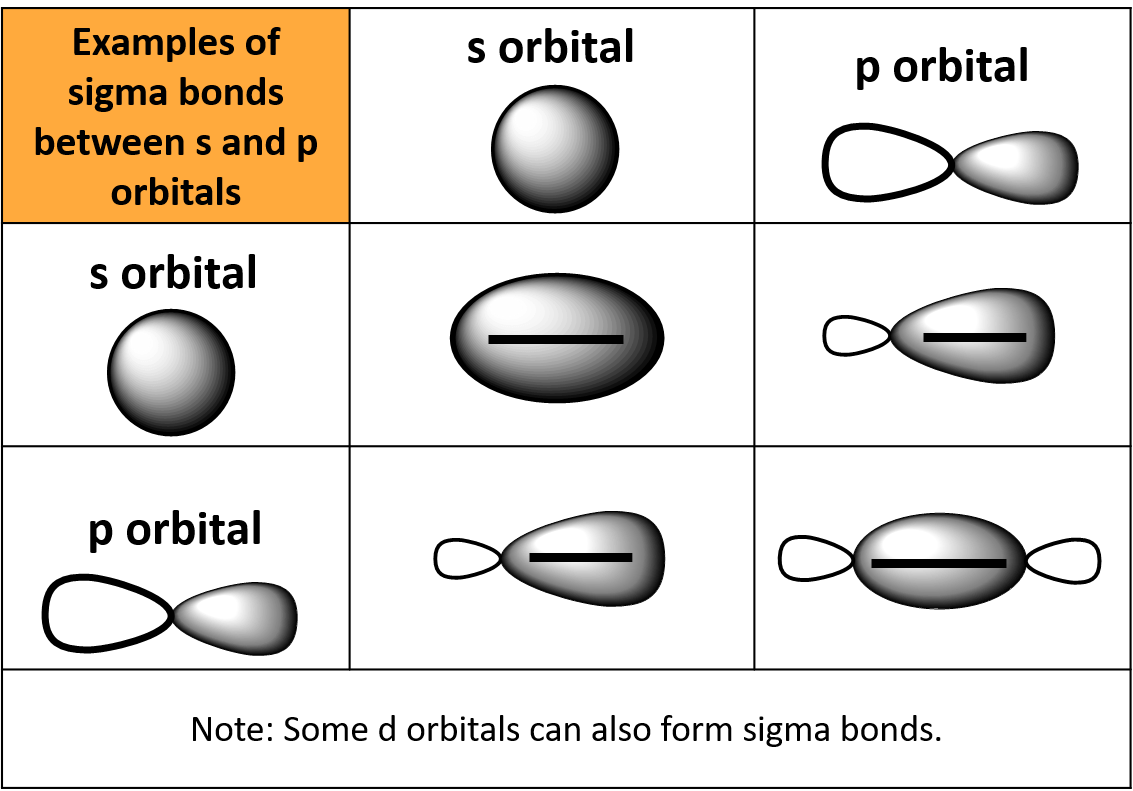
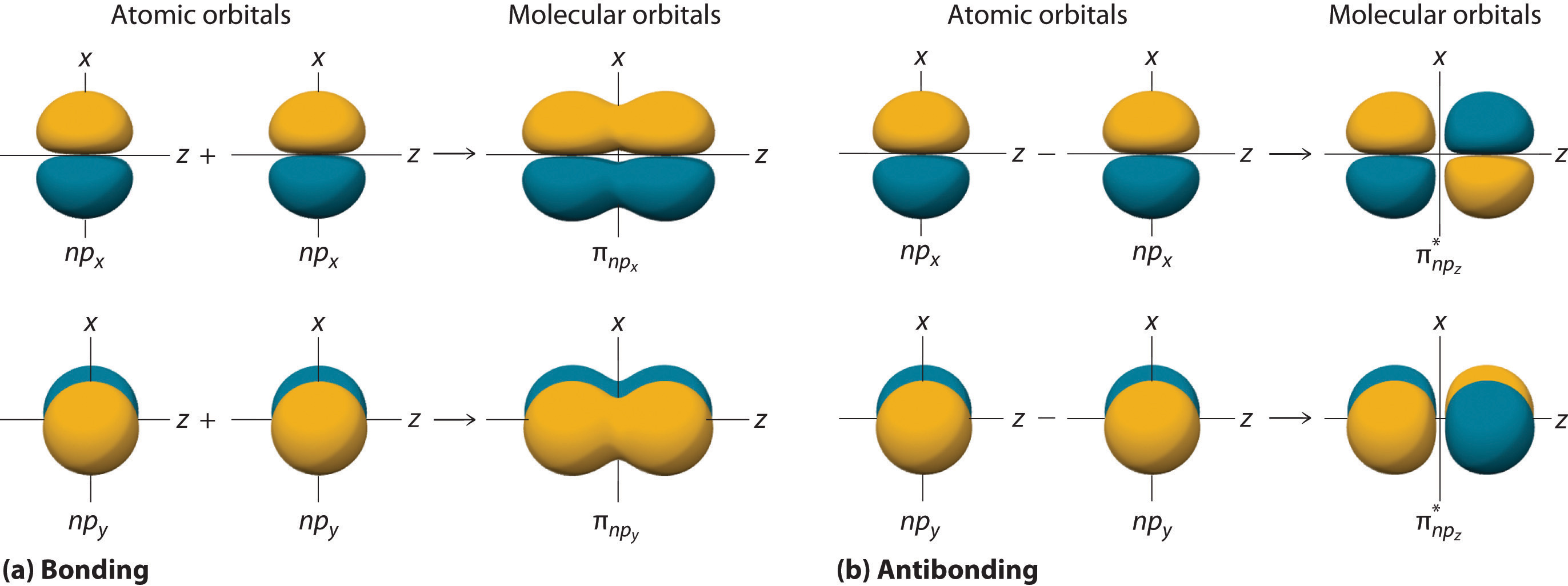
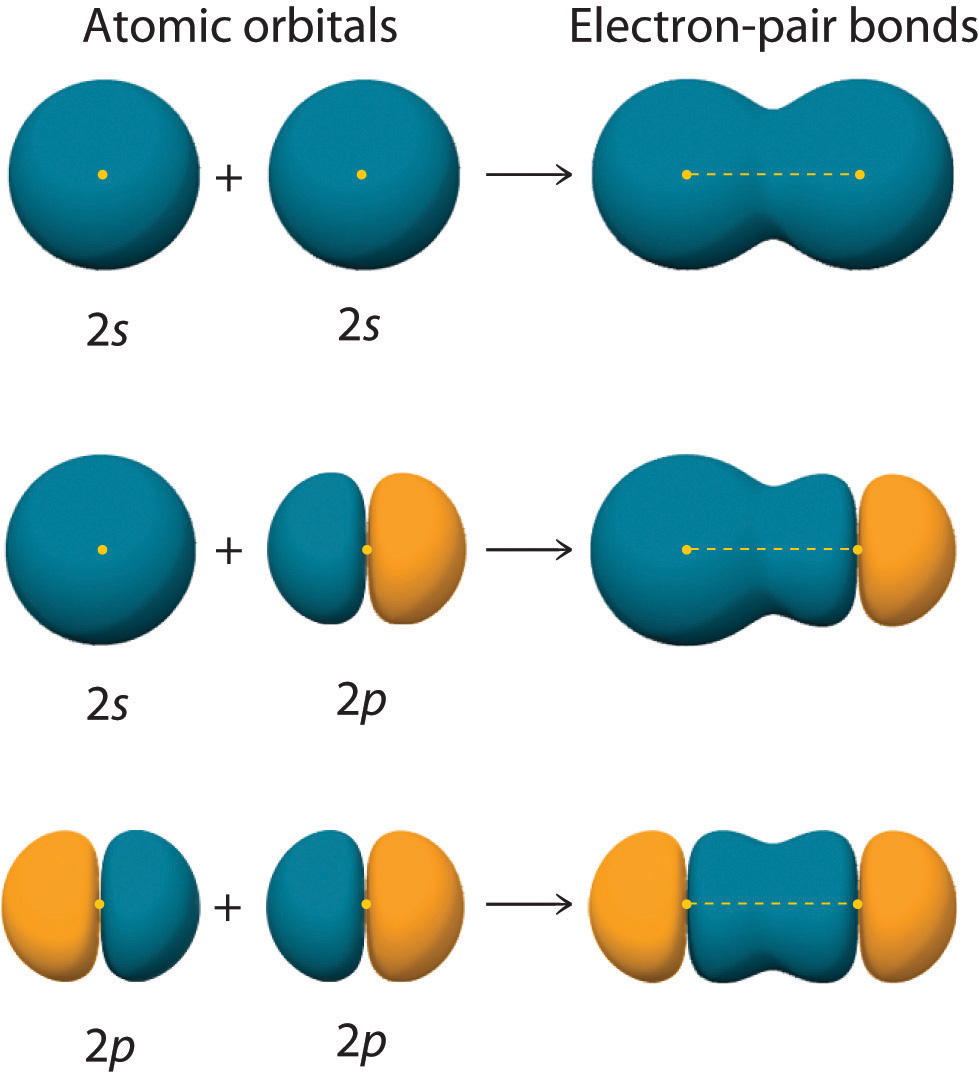
Can P Orbitals Form Sigma Bonds - Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A pi (π) orbital is one that has one node. This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the.
A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. This type of overlap allows the electron density to be concentrated along.
This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the.
Solved When two p atomic orbitals combine, two different
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. This type of overlap allows the electron density to be concentrated along. A pi (π) orbital is one that has one node. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can.
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A sigma bond can also be formed by the overlap of two p orbitals. This type of overlap allows the electron density to be concentrated along. A pi (π) orbital is one that has one node. The covalent bond in molecular.
Alkenes (ALevel) ChemistryStudent
A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. A sigma bond can also be formed by the overlap of two p orbitals. This type of overlap allows the electron density to be concentrated along. The covalent bond in molecular.
Pi Bond And Sigma Bond How to count sigma and pi bonds Quora 3
This type of overlap allows the electron density to be concentrated along. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can.
8 Drawing Molecular Orbital Diagrams — Flux Science
A pi (π) orbital is one that has one node. This type of overlap allows the electron density to be concentrated along. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are.
9.3 Molecular Orbital Theory Chemistry LibreTexts
This type of overlap allows the electron density to be concentrated along. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can also be formed by the overlap of two p orbitals..
A π bond is formed by the overlap of
The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. This type of overlap allows the electron density to be concentrated along. A sigma bond can also be formed by the overlap of two p orbitals. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are.
Sigma和π键聪明的数学和科学Wiki
A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. This type of overlap allows the electron density to be concentrated along. A pi (π) orbital is one that has one node. Any two orbitals (s, p, or d) that are.
sigma /r/okbuddyretard OkBuddyRetard Know Your Meme
A sigma bond can also be formed by the overlap of two p orbitals. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A pi (π) orbital is one that has one node. This type.
2p Orbitals
A pi (π) orbital is one that has one node. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. A sigma bond can also be formed by the overlap of two p orbitals. This type of overlap allows the electron density to be concentrated along. Any two orbitals (s, p, or d) that are.
A Pi (Π) Orbital Is One That Has One Node.
A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, f 2, is a sigma bond formed by the. Any two orbitals (s, p, or d) that are oriented along the bonding axis can form a sigma bond. This type of overlap allows the electron density to be concentrated along.